Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation
- PMID: 18818211
- PMCID: PMC2662218
- DOI: 10.1074/jbc.M801844200
Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation
Abstract
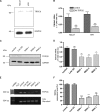
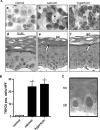
The protective epithelial barrier in our skin undergoes constant regulation, whereby the balance between differentiation and proliferation of keratinocytes plays a major role. Impaired keratinocyte differentiation and proliferation are key elements in the pathophysiology of several important dermatological diseases, including atopic dermatitis and psoriasis. Ca(2+) influx plays an essential role in this process presumably mediated by different transient receptor potential (TRP) channels. However, investigating their individual role was hampered by the lack of specific stimulators or inhibitors. Because we have recently identified hyperforin as a specific TRPC6 activator, we investigated the contribution of TRPC6 to keratinocyte differentiation and proliferation. Like the endogenous differentiation stimulus high extracellular Ca(2+) concentration ([Ca(2+)](o)), hyperforin triggers differentiation in HaCaT cells and in primary cultures of human keratinocytes by inducing Ca(2+) influx via TRPC6 channels and additional inhibition of proliferation. Knocking down TRPC6 channels prevents the induction of Ca(2+)- and hyperforin-induced differentiation. Importantly, TRPC6 activation is sufficient to induce keratinocyte differentiation similar to the physiological stimulus [Ca(2+)](o). Therefore, TRPC6 activation by hyperforin may represent a new innovative therapeutic strategy in skin disorders characterized by altered keratinocyte differentiation.
Figures


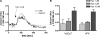




Similar articles
-
Hyperforin activates gene transcription involving transient receptor potential C6 channels.Biochem Pharmacol. 2017 Apr 1;129:96-107. doi: 10.1016/j.bcp.2017.01.007. Epub 2017 Jan 19. Biochem Pharmacol. 2017. PMID: 28110963
-
Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators--identification of a novel pharmacophore.Mol Pharmacol. 2010 Mar;77(3):368-77. doi: 10.1124/mol.109.057513. Epub 2009 Dec 14. Mol Pharmacol. 2010. PMID: 20008516
-
The TRPC6 channel activator hyperforin induces the release of zinc and calcium from mitochondria.J Neurochem. 2010 Jan;112(1):204-13. doi: 10.1111/j.1471-4159.2009.06446.x. Epub 2009 Oct 21. J Neurochem. 2010. PMID: 19845832
-
[Cellular and molecular effects of the antidepressant hyperforin on brain cells: Review of the literature].Encephale. 2014 Apr;40(2):108-13. doi: 10.1016/j.encep.2013.03.004. Epub 2013 Jun 29. Encephale. 2014. PMID: 23816060 Review. French.
-
[Mechanism of cardiac hypertrophy via diacylglycerol-sensitive TRPC channels].Yakugaku Zasshi. 2010 Mar;130(3):295-302. doi: 10.1248/yakushi.130.295. Yakugaku Zasshi. 2010. PMID: 20190513 Review. Japanese.
Cited by
-
Polysaccharides of St. John's Wort Herb Stimulate NHDF Proliferation and NEHK Differentiation via Influence on Extracellular Structures and Signal Pathways.Adv Pharmacol Sci. 2012;2012:304317. doi: 10.1155/2012/304317. Epub 2012 Jul 17. Adv Pharmacol Sci. 2012. PMID: 22848211 Free PMC article.
-
TRP channels in skin: from physiological implications to clinical significances.Biophysics (Nagoya-shi). 2015 Feb 13;11:17-24. doi: 10.2142/biophysics.11.17. eCollection 2015. Biophysics (Nagoya-shi). 2015. PMID: 27493510 Free PMC article. Review.
-
Reversal of murine epidermal atrophy by topical modulation of calcium signaling.J Invest Dermatol. 2014 Jun;134(6):1599-1608. doi: 10.1038/jid.2013.524. Epub 2013 Dec 6. J Invest Dermatol. 2014. PMID: 24317393
-
TRP channels in the skin.Br J Pharmacol. 2014 May;171(10):2568-81. doi: 10.1111/bph.12569. Br J Pharmacol. 2014. PMID: 24372189 Free PMC article. Review.
-
Trp channels and itch.Semin Immunopathol. 2016 May;38(3):293-307. doi: 10.1007/s00281-015-0530-4. Epub 2015 Sep 18. Semin Immunopathol. 2016. PMID: 26385480 Free PMC article. Review.
References
-
- Lowes, M. A., Bowcock, A. M., and Krueger, J. G. (2007) Nature 445 866–873 - PubMed
-
- Bovenschen, H. J., Seyger, M. M., and Van De Kerkhof, P. C. (2005) Br. J. Dermatol. 153 72–78 - PubMed
-
- Williams, H. C. (2005) N. Engl. J. Med. 352 2314–2324 - PubMed
-
- Proksch, E., Folster-Holst, R., and Jensen, J. M. (2006) J. Dermatol. Sci. 43 159–169 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

