Na+,K+-ATPase is modulated by angiotensin II in diabetic rat kidney--another reason for diabetic nephropathy?
- PMID: 18818245
- PMCID: PMC2655384
- DOI: 10.1113/jphysiol.2008.156703
Na+,K+-ATPase is modulated by angiotensin II in diabetic rat kidney--another reason for diabetic nephropathy?
Abstract
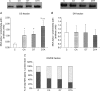
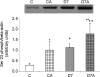
Angiotensin II (ANGII) plays a central role in the enhanced sodium reabsorption in early type 1 diabetes in man and in streptozotocin-induced (STZ) diabetic rats. This study investigates the effect of untreated STZ-diabetes leading to diabetic nephropathy in combination with ANGII treatment, on the abundance and localization of the renal Na(+),K(+)-ATPase (NKA), a major contributor of renal sodium handling. After 7 weeks of STZ-diabetes (i.v. 65 mg kg(-1)) a subgroup of control (C) and diabetic (D7) Wistar rats were treated with ANGII (s.c. minipump 33 microg kg(-1) h(-1) for 24 h; CA and D7A). We measured renal function and mRNA expression, protein level, Serin23 phosphorylation, subcellular distribution, and enzyme activity of NKA alpha-1 subunit in the kidney cortex. Diabetes increased serum creatinine and urea nitrogen levels (C versus D7), as did ANGII (C versus CA, D7 versus D7A). Both diabetes (C versus D7) and ANGII increased NKA alpha-1 protein level and enzyme activity (C versus CA, D7 versus D7A). Furthermore, the combination led to an additive increase (D7 versus D7A, CA versus D7A). NKA alpha-1 Ser23 phosphorylation was higher both in D7 and ANGII-treated rats in the non-cytoskeletal fraction, while no signal was detected in the cytoskeletal fraction. Control kidneys showed NKA alpha-1 immunopositivity on the basolateral membrane of proximal tubular cells, while both D7 and ANGII broadened NKA immunopositivity towards the cytoplasm. Our study demonstrates that diabetes mellitus (DM) increases the mRNA expression, protein level, Ser23 phosphorylation and enzyme activity of renal NKA, which is further elevated by ANGII. Despite an increase in total NKA quantity in diabetic nephropathy, the redistribution to the cystosol suggests the Na(+) pump is no longer functional. ANGII also caused translocation from the basolateral membrane, thus in diabetic states where ANGII level is acutely elevated, the loss of NKA will be exacerbated. This provides another mechanism by which ANGII blockade is likely to be protective.
Figures


Comment in
-
Is renal Na+,K+-ATPase a new target for renin-angiotensin blocking agents in diabetic nephropathy?J Physiol. 2008 Nov 15;586(22):5283. doi: 10.1113/jphysiol.2008.164723. J Physiol. 2008. PMID: 19011129 Free PMC article. No abstract available.
References
-
- American Diabetes Association. Standards of medical care in diabetes (Position Statement) Diabetes Care. 2005;28:S4–S36. - PubMed
-
- Andersson RM, Aizman O, Aperia A, Brismar H. Modulation of Na+,K+-ATPase activity is of importance for RVD. Acta Physiol Scand. 2004;180:329–334. - PubMed
-
- Aufricht C, Bidmon B, Ruffingshofer D, Regele H, Herkner K, Siegel NJ, Kashgarian M, Van Why S. Ischemic conditioning prevents Na,K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res. 2002;51:722–727. - PubMed
-
- Azuma KK, Hensley CB, Tang MJ, McDonough AA. Thyroid hormone specifically regulates skeletal muscle Na+-K+-ATPase alpha 2- and beta 2-isoforms. Am J Physiol Cell Physiol. 1993;265:C680–C687. - PubMed
-
- Bertuccio CA, Arrizurieta EE, Ibarra FR, Martín RS. Mechanisms of PKC-dependent Na+ K+ ATPase phosphorylation in the rat kidney with chronic renal failure. Ren Fail. 2007;29:13–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

