Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation
- PMID: 18818300
- PMCID: PMC2646529
- DOI: 10.1210/en.2008-0213
Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation
Abstract
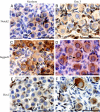
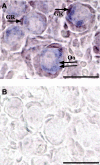
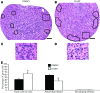
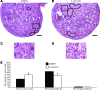
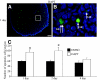
Notch signaling directs cell fate during embryogenesis by influencing cell proliferation, differentiation, and apoptosis. Notch genes are expressed in the adult mouse ovary, and roles for Notch in regulating folliculogenesis are beginning to emerge from mouse genetic models. We investigated how Notch signaling might influence the formation of primordial follicles. Follicle assembly takes place when germ cell syncytia within the ovary break down and germ cells are encapsulated by pregranulosa cells. In the mouse, this occurs during the first 4-5 d of postnatal life. The expression of Notch family genes in the neonatal mouse ovary was determined through RT-PCR measurements. Jagged1, Notch2, and Hes1 transcripts were the most abundantly expressed ligand, receptor, and target gene, respectively. Jagged1 and Hey2 mRNAs were up-regulated over the period of follicle formation. Localization studies demonstrated that JAGGED1 is expressed in germ cells prior to follicle assembly and in the oocytes of primordial follicles. Pregranulosa cells that surround germ cell nests express HES1. In addition, pregranulosa cells of primordial follicles expressed NOTCH2 and Hey2 mRNA. We used an ex vivo ovary culture system to assess the requirement for Notch signaling during early follicle development. Newborn ovaries cultured in the presence of gamma-secretase inhibitors, compounds that attenuate Notch signaling, had a marked reduction in primordial follicles compared with vehicle-treated ovaries, and there was a corresponding increase in germ cells that remained within nests. These data support a functional role for Notch signaling in regulating primordial follicle formation.
Figures
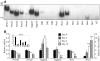


References
-
- McGee EA, Hsueh AJ 2000 Initial and cyclic recruitment of ovarian follicles. Endocr Rev 21:200–214 - PubMed
-
- Molyneaux KA, Stallock J, Schaible K, Wylie C 2001 Time-lapse analysis of living mouse germ cell migration. Dev Biol 240:488–498 - PubMed
-
- Pepling ME, Spradling AC 1998 Female mouse germ cells form synchronously dividing cysts. Development 125:3323–3328 - PubMed
-
- Pepling ME, Spradling AC 2001 Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234:339–351 - PubMed
-
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK 2006 Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol 298:132–148 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

