Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells
- PMID: 18818306
- PMCID: PMC2567482
- DOI: 10.1073/pnas.0802555105
Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells
Abstract
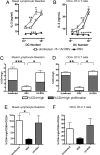
Respiratory syncytial virus (RSV) infection is one of the leading causes of infant hospitalization and a major health and economic burden worldwide. Infection with this virus induces an exacerbated innate proinflammatory immune response characterized by abundant immune cell infiltration into the airways and lung tissue damage. RSV also impairs the induction of an adequate adaptive T cell immune response, which favors virus pathogenesis. Unfortunately, to date there are no efficient vaccines against this virus. Recent in vitro and in vivo studies suggest that RSV infection can prevent T cell activation, a phenomenon attributed in part to cytokines and chemokines secreted by RSV-infected cells. Efficient immunity against viruses is promoted by dendritic cells (DCs), professional antigen-presenting cells, that prime antigen-specific helper and cytotoxic T cells. Therefore, it would be to the advantage of RSV to impair DC function and prevent the induction of T cell immunity. Here, we show that, although RSV infection induces maturation of murine DCs, these cells are rendered unable to activate antigen-specific T cells. Inhibition of T cell activation by RSV was observed independently of the type of TCR ligand on the DC surface and applied to cognate-, allo-, and superantigen stimulation. As a result of exposure to RSV-infected DCs, T cells became unresponsive to subsequent TCR engagement. RSV-mediated impairment in T cell activation required DC-T cell contact and involved inhibition of immunological synapse assembly among these cells. Our data suggest that impairment of immunological synapse could contribute to RSV pathogenesis by evading adaptive immunity and reducing T cell-mediated virus clearance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



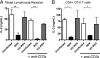

Similar articles
-
Contribution of Fcγ receptors to human respiratory syncytial virus pathogenesis and the impairment of T-cell activation by dendritic cells.Immunology. 2016 Jan;147(1):55-72. doi: 10.1111/imm.12541. Epub 2015 Nov 6. Immunology. 2016. PMID: 26451966 Free PMC article.
-
Lack of Activation Marker Induction and Chemokine Receptor Switch in Human Neonatal Myeloid Dendritic Cells in Response to Human Respiratory Syncytial Virus.J Virol. 2019 Oct 29;93(22):e01216-19. doi: 10.1128/JVI.01216-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31484754 Free PMC article.
-
Impairment of T cell immunity by the respiratory syncytial virus: targeting virulence mechanisms for therapy and prophylaxis.Curr Med Chem. 2009;16(34):4609-25. doi: 10.2174/092986709789760724. Curr Med Chem. 2009. PMID: 19903147 Review.
-
Autophagy-mediated dendritic cell activation is essential for innate cytokine production and APC function with respiratory syncytial virus responses.J Immunol. 2011 Oct 15;187(8):3953-61. doi: 10.4049/jimmunol.1100524. Epub 2011 Sep 12. J Immunol. 2011. PMID: 21911604 Free PMC article.
-
Host immunity during RSV pathogenesis.Int Immunopharmacol. 2008 Oct;8(10):1320-9. doi: 10.1016/j.intimp.2008.03.012. Epub 2008 Apr 14. Int Immunopharmacol. 2008. PMID: 18687294 Review.
Cited by
-
Biology of human respiratory syncytial virus: Current perspectives in immune response and mechanisms against the virus.Virus Res. 2024 Dec;350:199483. doi: 10.1016/j.virusres.2024.199483. Epub 2024 Oct 18. Virus Res. 2024. PMID: 39396572 Free PMC article. Review.
-
Increased Heme Oxygenase 1 Expression upon a Primary Exposure to the Respiratory Syncytial Virus and a Secondary Mycobacterium bovis Infection.Antioxidants (Basel). 2022 Jul 26;11(8):1453. doi: 10.3390/antiox11081453. Antioxidants (Basel). 2022. PMID: 35892656 Free PMC article.
-
Modulation of host adaptive immunity by hRSV proteins.Virulence. 2014;5(7):740-51. doi: 10.4161/viru.32225. Virulence. 2014. PMID: 25513775 Free PMC article. Review.
-
Heme Oxygenase-1 Expression in Dendritic Cells Contributes to Protective Immunity against Herpes Simplex Virus Skin Infection.Antioxidants (Basel). 2023 May 29;12(6):1170. doi: 10.3390/antiox12061170. Antioxidants (Basel). 2023. PMID: 37371900 Free PMC article.
-
Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection.Virulence. 2017 Aug 18;8(6):685-704. doi: 10.1080/21505594.2016.1265725. Epub 2016 Dec 2. Virulence. 2017. PMID: 27911218 Free PMC article. Review.
References
-
- Shay D-K, et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. J Am Med Assoc. 1999;282:1440–1446. - PubMed
-
- Glezen W-P, Taber L-H, Frank A-L, Kasel J-A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. - PubMed
-
- Henderson F-W, Collier A-M, Clyde W-A, Jr, Denny F-W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. - PubMed
-
- Hall C-B, Walsh E-E, Long C-E, Schnabel K-C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. - PubMed
-
- Zeng R, et al. Long-lasting balanced immunity and protective efficacy against respiratory syncytial virus in mice induced by a recombinant protein G1F/M2. Vaccine. 2007;25:7422–7428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

