Hyaluronan-mediated leukocyte adhesion and dextran sulfate sodium-induced colitis are attenuated in the absence of signal transducer and activator of transcription 1
- PMID: 18818378
- PMCID: PMC2570126
- DOI: 10.2353/ajpath.2008.080444
Hyaluronan-mediated leukocyte adhesion and dextran sulfate sodium-induced colitis are attenuated in the absence of signal transducer and activator of transcription 1
Abstract
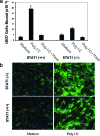
Inflammatory bowel disease is a chronic inflammatory condition of the intestinal mucosa whose etiology is unclear but is likely to be multifactorial. We have shown previously that an increased amount of hyaluronan (HA) is present both in the inflamed mucosa of inflammatory bowel disease patients and in isolated human cells after polyI:C treatment. The signal transducer and activator of transcription (STAT)1 protein plays an important role in many signaling pathways that are associated with inflammation. We therefore investigated the role of STAT1 in adhesive interactions that occur between leukocytes and polyI:C-induced mucosal smooth muscle cells (M-SMCs). Activation of STAT1 was observed after the polyI:C treatment of M-SMCs. Specific phosphorylation of tyrosine and serine residues of STAT1 was observed in polyI:C-treated, but not untreated, M-SMC cultures. To evaluate further the role of STAT1, a corresponding STAT-1-null mouse was used. PolyI:C-induced, HA-mediated leukocyte adhesion to colon SMCs from STAT1-null mice was significantly decreased compared with that from wild-type control mice. In vivo, using the dextran sulfate sodium-induced model of colon inflammation, both tissue damage and HA deposition were attenuated in STAT1-null mice compared with that in wild-type control mice. Additionally, the inter-alpha-trypsin inhibitor (IalphaI), a proteoglycan essential for facilitating leukocyte binding to the HA matrix, was reduced in STAT1-null mice. Together, these results demonstrate that STAT1 plays an important role in HA-mediated inflammatory processes.
Figures




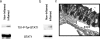
References
-
- Beagley KW, Elson CO. Cells and cytokines in mucosal immunity and inflammation. Gastroenterol Clin North Am. 1992;21:347–366. - PubMed
-
- Becker C, Dornhoff H, Neufert C, Fantini MC, Wirtz S, Huebner S, Nikolaev A, Lehr HA, Murphy AJ, Valenzuela DM, Yancopoulos GD, Galle PR, Karow M, Neurath MF. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–2764. - PubMed
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. - PubMed
-
- Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. - PubMed
-
- Fiocchi C, Fukushima K, Strong SA, Ina K. Pitfalls in cytokine analysis in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10(Suppl 2):61–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

