Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines
- PMID: 18818437
- PMCID: PMC2758553
- DOI: 10.1634/stemcells.2008-0519
Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines
Abstract
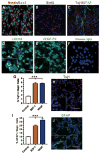
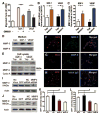
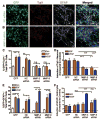
Adult neurogenesis is regulated by both intrinsic programs and extrinsic stimuli. The enhanced proliferation of adult neural stem/progenitor cells (aNPCs) in the subventricular zone and the migration of neuroblasts toward the ischemic region in adult brains present a unique challenge as well as an opportunity to understand the molecular mechanisms underlying the extrinsic cue-induced neurogenic responses. Matrix metalloproteinases (MMPs) are a family of proteinases known to play a role in extracellular matrix remodeling and cell migration. However, their presence in aNPCs and their potential function in injury-induced aNPC migration remain largely unexplored. Here we demonstrate that in response to two injury-induced chemokines, stromal cell-derived factor 1 (SDF-1) and vascular endothelial growth factor, aNPCs differentiated into migratory cells that expressed increased levels of MMP-3 and MMP-9. Whereas differentiated neuroblasts and a subpopulation of astrocytes migrated toward the chemokines, undifferentiated progenitors did not migrate. Blocking the expression of MMP-3 or MMP-9 in aNPCs interfered with both the differentiation of aNPCs and chemokine-induced cell migration. Thus, endogenous MMPs expressed by aNPCs are important for mediating their neurogenic response to extrinsic signals.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures


References
-
- Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–132. - PubMed
-
- Thored P, Aridsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. - PubMed
-
- Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angio-poietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. - PubMed
-
- Robin AM, Zhang ZG, Wang L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

