The insulin-like growth factor system as a potential therapeutic target in gastrointestinal stromal tumors
- PMID: 18818517
- PMCID: PMC2626174
- DOI: 10.4161/cc.7.19.6760
The insulin-like growth factor system as a potential therapeutic target in gastrointestinal stromal tumors
Abstract
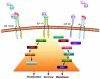
The majority of gastrointestinal stromal tumors (GISTs) are characterized by oncogenic gain-of-function mutations in the receptor tyrosine kinase (RTK) c-KIT with a minority in PDGFRalpha. Therapy for GISTs has been revolutionized by the use of the selective tyrosine kinase inhibitor imatinib mesylate (IM). For the subset (approximately 10-15%) of GISTs that lack oncogenic mutations in these receptors, the genetic changes driving tumorigenesis are unknown. We recently reported that the gene encoding the insulin-like growth factor 1 receptor (IGF-1R) is amplified in a subset of GISTs, and the IGF-1R protein is overexpressed in wild-type and pediatric GISTs. In this report we present a more complete picture of the involvement of components of the insulin-like growth factor-signaling pathway in the pathogenesis of GISTs. We also discuss how the IGF pathway may provide additional molecular targets for the treatment of GISTs that respond poorly to IM therapy.
Figures




References
-
- Corless CL, Heinrich MC. Molecular Pathobiology of Gastrointestinal Stromal Sarcomas. Annu Rev Pathol. 2007 - PubMed
-
- Tarn C, Godwin AK. The molecular pathogenesis of gastrointestinal stromal tumors. Clin Colorectal Cancer. 2006;6(Suppl 1):S7–17. - PubMed
-
- Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA, Demetri GD, Fletcher CD, Fletcher JA. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–21. - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
