IKKalpha, a critical regulator of epidermal differentiation and a suppressor of skin cancer
- PMID: 18818691
- PMCID: PMC2556095
- DOI: 10.1038/emboj.2008.196
IKKalpha, a critical regulator of epidermal differentiation and a suppressor of skin cancer
Abstract
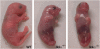
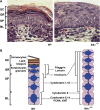
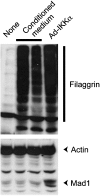
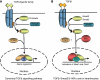
IkappaB kinase alpha (IKKalpha), one of the two catalytic subunits of the IKK complex involved in nuclear factor kappaB (NF-kappaB) activation, also functions as a molecular switch that controls epidermal differentiation. This unexpected function requires IKKalpha nuclear translocation but does not depend on its kinase activity, and is independent of NF-kappaB signalling. Ikkalpha(-/-) mice present with a hyperproliferative and undifferentiated epidermis characterized by complete absence of a granular layer and stratum corneum. Ikkalpha-deficient keratinocytes do not express terminal differentiation markers and continue to proliferate even when subjected to differentiation-inducing stimuli. This antiproliferative function of IKKalpha is also important for the suppression of squamous cell carcinogenesis. The exact mechanisms by which nuclear IKKalpha controls keratinocyte proliferation and differentiation remained mysterious for some time. Recent studies, however, have revealed that IKKalpha is a major cofactor in a TGFbeta-Smad2/3 signalling pathway that is Smad4 independent. This pathway controls cell cycle withdrawal during keratinocyte terminal differentiation. Although these are not the only functions of nuclear IKKalpha, this multifunctional protein is a key regulator of keratinocyte and epidermal differentiation and a critical suppressor of skin cancer.
Figures


References
-
- Ayer DE, Kretzner L, Eisenman RN (1993) Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell 72: 211–222 - PubMed
-
- Candi E, Dinsdale D, Rufini A, Salomoni P, Knight RA, Mueller M, Krammer PH, Melino G (2007) TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle 6: 274–285 - PubMed
-
- Candi E, Schmidt R, Melino G (2005) The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6: 328–340 - PubMed
-
- Chen CR, Kang Y, Siegel PM, Massague J (2002) E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell 110: 19–32 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

