A genomewide functional network for the laboratory mouse
- PMID: 18818725
- PMCID: PMC2527685
- DOI: 10.1371/journal.pcbi.1000165
A genomewide functional network for the laboratory mouse
Abstract
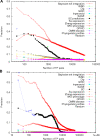
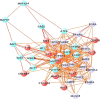
Establishing a functional network is invaluable to our understanding of gene function, pathways, and systems-level properties of an organism and can be a powerful resource in directing targeted experiments. In this study, we present a functional network for the laboratory mouse based on a Bayesian integration of diverse genetic and functional genomic data. The resulting network includes probabilistic functional linkages among 20,581 protein-coding genes. We show that this network can accurately predict novel functional assignments and network components and present experimental evidence for predictions related to Nanog homeobox (Nanog), a critical gene in mouse embryonic stem cell pluripotency. An analysis of the global topology of the mouse functional network reveals multiple biologically relevant systems-level features of the mouse proteome. Specifically, we identify the clustering coefficient as a critical characteristic of central modulators that affect diverse pathways as well as genes associated with different phenotype traits and diseases. In addition, a cross-species comparison of functional interactomes on a genomic scale revealed distinct functional characteristics of conserved neighborhoods as compared to subnetworks specific to higher organisms. Thus, our global functional network for the laboratory mouse provides the community with a key resource for discovering protein functions and novel pathway components as well as a tool for exploring systems-level topological and evolutionary features of cellular interactomes. To facilitate exploration of this network by the biomedical research community, we illustrate its application in function and disease gene discovery through an interactive, Web-based, publicly available interface at http://mouseNET.princeton.edu.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Jansen R, Yu H, Greenbaum D, Kluger Y, Krogan NJ, et al. A Bayesian networks approach for predicting protein-protein interactions from genomic data. Science. 2003;302:449–453. - PubMed
-
- Lee I, Date SV, Adai AT, Marcotte EM. A probabilistic functional network of yeast genes. Science. 2004;306:1555–1558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

