Distinct effects of IL-18 on the engraftment and function of human effector CD8 T cells and regulatory T cells
- PMID: 18818761
- PMCID: PMC2538560
- DOI: 10.1371/journal.pone.0003289
Distinct effects of IL-18 on the engraftment and function of human effector CD8 T cells and regulatory T cells
Abstract
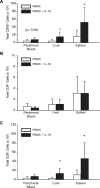
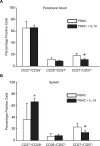
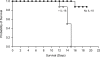
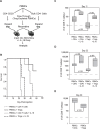
IL-18 has pleotropic effects on the activation of T cells during antigen presentation. We investigated the effects of human IL-18 on the engraftment and function of human T cell subsets in xenograft mouse models. IL-18 enhanced the engraftment of human CD8(+) effector T cells and promoted the development of xenogeneic graft versus host disease (GVHD). In marked contrast, IL-18 had reciprocal effects on the engraftment of CD4(+)CD25(+)Foxp3(+) regulatory T cells (Tregs) in the xenografted mice. Adoptive transfer experiments indicated that IL-18 prevented the suppressive effects of Tregs on the development of xenogeneic GVHD. The IL-18 results were robust as they were observed in two different mouse strains. In addition, the effects of IL-18 were systemic as IL-18 promoted engraftment and persistence of human effector T cells and decreased Tregs in peripheral blood, peritoneal cavity, spleen and liver. In vitro experiments indicated that the expression of the IL-18Ralpha was induced on both CD4 and CD8 effector T cells and Tregs, and that the duration of expression was less sustained on Tregs. These preclinical data suggest that human IL-18 may have use as an adjuvant for immune reconstitution after cytotoxic therapies, and to augment adoptive immunotherapy, donor leukocyte infusions, and vaccine strategies.
Conflict of interest statement
Figures


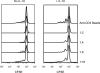
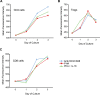
References
-
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. - PubMed
-
- Ushio S, Namba M, Okura T, Hattori K, Nukada Y, et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. - PubMed
-
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. - PubMed
-
- Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224. - PubMed
-
- Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

