A novel monoacylglycerol lipase inhibitor with analgesic and anti-inflammatory activity
- PMID: 18819796
- PMCID: PMC3712614
- DOI: 10.1016/j.bmcl.2008.09.039
A novel monoacylglycerol lipase inhibitor with analgesic and anti-inflammatory activity
Abstract
A variety of long chain 1,2-diamines and related compounds were synthesized and tested for their activity on fatty acid amide hydrolase (FAAH) and monoacyglycerol lipase (MGL). (2S,9Z)-Octadec-9-ene-1,2-diamine selectively inhibits MGL (K(i) 21.8 microM) without significantly affecting FAAH. This compound exhibited interesting in vivo analgesic and anti-inflammatory properties, suggesting that selective inhibitors of MGL may be valuable novel agents for the treatment of inflammatory pain.
Figures
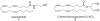


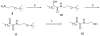



Similar articles
-
Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment.Pharmacol Res. 2013 Jan;67(1):94-109. doi: 10.1016/j.phrs.2012.10.013. Epub 2012 Nov 2. Pharmacol Res. 2013. PMID: 23127915 Free PMC article.
-
Fatty acid amide hydrolase inhibitors from virtual screening of the endocannabinoid system.J Med Chem. 2006 Jul 27;49(15):4650-6. doi: 10.1021/jm060394q. J Med Chem. 2006. PMID: 16854070
-
Fatty pain cures.Chem Biol. 2007 Dec;14(12):1311-2. doi: 10.1016/j.chembiol.2007.12.003. Chem Biol. 2007. PMID: 18096498
-
The Potential of Inhibitors of Endocannabinoid Metabolism for Drug Development: A Critical Review.Handb Exp Pharmacol. 2015;231:95-128. doi: 10.1007/978-3-319-20825-1_4. Handb Exp Pharmacol. 2015. PMID: 26408159 Review.
-
Overview of the chemical families of fatty acid amide hydrolase and monoacylglycerol lipase inhibitors.Curr Top Med Chem. 2008;8(3):247-67. doi: 10.2174/156802608783498005. Curr Top Med Chem. 2008. PMID: 18289091 Review.
Cited by
-
Identification by nuclear magnetic resonance spectroscopy of an active-site hydrogen-bond network in human monoacylglycerol lipase (hMGL): implications for hMGL dynamics, pharmacological inhibition, and catalytic mechanism.Mol Biosyst. 2010 Aug;6(8):1381-8. doi: 10.1039/c004515b. Epub 2010 May 12. Mol Biosyst. 2010. PMID: 20464001 Free PMC article.
-
Monoacylglycerol lipase - a target for drug development?Br J Pharmacol. 2012 Jul;166(5):1568-85. doi: 10.1111/j.1476-5381.2012.01950.x. Br J Pharmacol. 2012. PMID: 22428756 Free PMC article. Review.
-
The endocannabinoid system and pain.CNS Neurol Disord Drug Targets. 2009 Dec;8(6):403-21. doi: 10.2174/187152709789824660. CNS Neurol Disord Drug Targets. 2009. PMID: 19839937 Free PMC article. Review.
References
-
- Kokotos G. Endocannabinoids. In: Kokotos G, Nicolaou A, editors. Bioactive Lipids. The Oily Press; Bridgewater, England: 2004. p. 245.
-
- Lambert DM, Fowler CJ. J. Med. Chem. 2005;48:5059. - PubMed
-
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminsky NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Biochem. Pharmacol. 1995;50:83. - PubMed
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem. Biophys. Res. Commun. 1995;215:89. - PubMed
- Stella N, Schweitzer P, Piomelli D. Nature. 1997;388:773. - PubMed
-
- Piomelli D. Curr. Opin. Investig. Drugs. 2005;6:672. - PubMed
- Di Marzo V, Bifulco M, De Petrocallis L. Nat. Rev. Drug Disc. 2004;3:771. - PubMed
- Makriyannis A, Mechoulam R, Piomelli D. 2005. Neuropharmacology. 48:1068. - PubMed
- Bahr BA, Karanian DA, Makanji SS, Makriyannis A. Expert Opin. Investig. Drugs. 2006;15:351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

