Projection structure of a member of the amino acid/polyamine/organocation transporter superfamily
- PMID: 18819925
- PMCID: PMC2662254
- DOI: 10.1074/jbc.M806917200
Projection structure of a member of the amino acid/polyamine/organocation transporter superfamily
Abstract
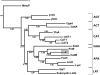
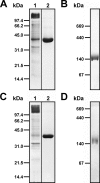
The L-arginine/agmatine antiporter AdiC is a key component of the arginine-dependent extreme acid resistance system of Escherichia coli. Phylogenetic analysis indicated that AdiC belongs to the amino acid/polyamine/organocation (APC) transporter superfamily having sequence identities of 15-17% to eukaryotic and human APC transporters. For functional and structural characterization, we cloned, overexpressed, and purified wild-type AdiC and the point mutant AdiC-W293L, which is unable to bind and consequently transport L-arginine. Purified detergent-solubilized AdiC particles were dimeric. Reconstitution experiments yielded two-dimensional crystals of AdiC-W293L diffracting beyond 6 angstroms resolution from which we determined the projection structure at 6.5 angstroms resolution. The projection map showed 10-12 density peaks per monomer and suggested mainly tilted helices with the exception of one distinct perpendicular membrane spanning alpha-helix. Comparison of AdiC-W293L with the projection map of the oxalate/formate antiporter from Oxalobacter formigenes, a member from the major facilitator superfamily, indicated different structures. Thus, two-dimensional crystals of AdiC-W293L yielded the first detailed view of a transport protein from the APC superfamily at sub-nanometer resolution.
Figures



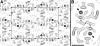

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

