The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers
- PMID: 18820677
- PMCID: PMC2597643
- DOI: 10.1038/nature07338
The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers
Abstract
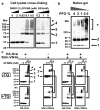
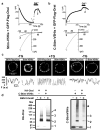
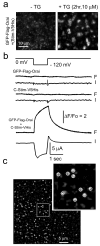
Ca(2+)-release-activated Ca(2+) (CRAC) channels underlie sustained Ca(2+) signalling in lymphocytes and numerous other cells after Ca(2+) liberation from the endoplasmic reticulum (ER). RNA interference screening approaches identified two proteins, Stim and Orai, that together form the molecular basis for CRAC channel activity. Stim senses depletion of the ER Ca(2+) store and physically relays this information by translocating from the ER to junctions adjacent to the plasma membrane, and Orai embodies the pore of the plasma membrane calcium channel. A close interaction between Stim and Orai, identified by co-immunoprecipitation and by Förster resonance energy transfer, is involved in the opening of the Ca(2+) channel formed by Orai subunits. Most ion channels are multimers of pore-forming subunits surrounding a central channel, which are preassembled in the ER and transported in their final stoichiometry to the plasma membrane. Here we show, by biochemical analysis after cross-linking in cell lysates and intact cells and by using non-denaturing gel electrophoresis without cross-linking, that Orai is predominantly a dimer in the plasma membrane under resting conditions. Moreover, single-molecule imaging of green fluorescent protein (GFP)-tagged Orai expressed in Xenopus oocytes showed predominantly two-step photobleaching, again consistent with a dimeric basal state. In contrast, co-expression of GFP-tagged Orai with the carboxy terminus of Stim as a cytosolic protein to activate the Orai channel without inducing Ca(2+) store depletion or clustering of Orai into punctae yielded mostly four-step photobleaching, consistent with a tetrameric stoichiometry of the active Orai channel. Interaction with the C terminus of Stim thus induces Orai dimers to dimerize, forming tetramers that constitute the Ca(2+)-selective pore. This represents a new mechanism in which assembly and activation of the functional ion channel are mediated by the same triggering molecule.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

