Origin of correlated activity between parasol retinal ganglion cells
- PMID: 18820692
- PMCID: PMC2575139
- DOI: 10.1038/nn.2199
Origin of correlated activity between parasol retinal ganglion cells
Abstract
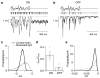
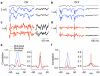
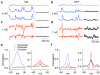
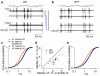
Cells throughout the CNS have synchronous activity patterns; that is, a cell's probability of generating an action potential depends both on its firing rate and on the occurrence of action potentials in surrounding cells. The mechanisms producing synchronous or correlated activity are poorly understood despite its prevalence and potential effect on neural coding. We found that neighboring parasol ganglion cells in primate retina received strongly correlated synaptic input in the absence of modulated light stimuli. This correlated variability appeared to arise through the same circuits that provide uncorrelated synaptic input. In addition, ON, but not OFF, parasol cells were coupled electrically. Correlated variability in synaptic input, however, dominated correlations in the parasol spike outputs and shared variability in the timing of action potentials generated by neighboring cells. These results provide a mechanistic picture of how correlated activity is produced in a population of neurons that are critical for visual perception.
Figures



References
-
- Usrey WM, Reid RC. Synchronous activity in the visual system. Annu Rev Physiol. 1999;61:435–456. - PubMed
-
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–366. - PubMed
-
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270:1207–1210. - PubMed
-
- Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci. 1998;1:501–507. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

