Transport mechanisms of carnosine in SKPT cells: contribution of apical and basolateral membrane transporters
- PMID: 18820998
- PMCID: PMC2913304
- DOI: 10.1007/s11095-008-9726-9
Transport mechanisms of carnosine in SKPT cells: contribution of apical and basolateral membrane transporters
Abstract
Purpose: The aim of this study was to investigate the transport properties of carnosine in kidney using SKPT cell cultures as a model of proximal tubular transport, and to isolate the functional activities of renal apical and basolateral transporters in this process.
Methods: The membrane transport kinetics of 10 microM [3H]carnosine was studied in SKPT cells as a function of time, pH, potential inhibitors and substrate concentration. A cellular compartment model was constructed in which the influx, efflux and transepithelial clearances of carnosine were determined. Peptide transporter expression was probed by RT-PCR.
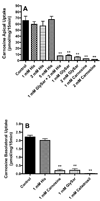
Results: Carnosine uptake was 15-fold greater from the apical than basolateral surface of SKPT cells. However, the apical-to-basolateral transepithelial transport of carnosine was severely rate-limited by its cellular efflux across the basolateral membrane. The high-affinity, proton-dependence, concentration-dependence and inhibitor specificity of carnosine supports the contention that PEPT2 is responsible for its apical uptake. In contrast, the basolateral transporter is saturable, inhibited by PEPT2 substrates but non-concentrative, thereby, suggesting a facilitative carrier.
Conclusions: Carnosine is expected to have a substantial cellular accumulation in kidney but minimal tubular reabsorption in blood because of its high influx clearance across apical membranes by PEPT2 and very low efflux clearance across basolateral membranes.
Figures






Similar articles
-
Carnosine uptake in rat choroid plexus primary cell cultures and choroid plexus whole tissue from PEPT2 null mice.J Neurochem. 2004 Apr;89(2):375-82. doi: 10.1111/j.1471-4159.2004.02333.x. J Neurochem. 2004. PMID: 15056281
-
Mechanism of intestinal absorption and renal reabsorption of an orally active ace inhibitor: uptake and transport of fosinopril in cell cultures.Drug Metab Dispos. 2001 Oct;29(10):1307-15. Drug Metab Dispos. 2001. PMID: 11560874
-
Characterization of rPEPT2-mediated Gly-Sar transport parameters in the rat kidney proximal tubule cell line SKPT-0193 cl.2 cultured in basic growth media.Mol Pharm. 2005 Mar-Apr;2(2):98-108. doi: 10.1021/mp049892q. Mol Pharm. 2005. PMID: 15804184
-
PEPT2-mediated transport of 5-aminolevulinic acid and carnosine in astrocytes.Brain Res. 2006 Nov 29;1122(1):18-23. doi: 10.1016/j.brainres.2006.09.013. Epub 2006 Oct 10. Brain Res. 2006. PMID: 17034769 Free PMC article.
-
[Molecular and cell biological analyses for intestinal absorption and renal excretion of drugs].Yakugaku Zasshi. 1997 Aug;117(8):522-41. doi: 10.1248/yakushi1947.117.8_522. Yakugaku Zasshi. 1997. PMID: 9306727 Review. Japanese.
Cited by
-
Carnosine treatment for gulf war illness: a randomized controlled trial.Glob J Health Sci. 2013 Feb 4;5(3):69-81. doi: 10.5539/gjhs.v5n3p69. Glob J Health Sci. 2013. PMID: 23618477 Free PMC article. Clinical Trial.
-
Dipeptides in CSF and plasma: diagnostic and therapeutic potential in neurological diseases.Amino Acids. 2024 Dec 13;57(1):2. doi: 10.1007/s00726-024-03434-1. Amino Acids. 2024. PMID: 39673003 Free PMC article.
-
Influence of genetic knockout of Pept2 on the in vivo disposition of endogenous and exogenous carnosine in wild-type and Pept2 null mice.Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R986-91. doi: 10.1152/ajpregu.90744.2008. Epub 2009 Feb 18. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19225147 Free PMC article.
-
Predicting Clearance Mechanism in Drug Discovery: Extended Clearance Classification System (ECCS).Pharm Res. 2015 Dec;32(12):3785-802. doi: 10.1007/s11095-015-1749-4. Epub 2015 Jul 9. Pharm Res. 2015. PMID: 26155985
-
THE EVALUATION OF PEPTIDE/HISTIDINE TRANSPORTER 1 (PHT1) FUNCTION: UPTAKE KINETICS UTILIZING A COS-7 STABLY TRANSFECTED CELL LINE.Rev Mex Cienc Farm. 2011 Oct;42(4):57-65. Rev Mex Cienc Farm. 2011. PMID: 23888104 Free PMC article.
References
-
- Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447:610–618. - PubMed
-
- Herrera-Ruiz D, Knipp GT. Current perspectives on established and putative mammalian oligopeptide transporters. J Pharm Sci. 2003;92:691–714. - PubMed
-
- Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. - PubMed
-
- Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC., 3rd Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol. 1999;276:F658–F665. - PubMed
-
- Groneberg DA, Döring F, Eynott PR, Fischer A, Daniel H. Intestinal peptide transport: Ex vivo uptake studies and localization of peptide carrier PEPT1. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G697–G704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

