Molecular targeted therapies in hepatocellular carcinoma
- PMID: 18821591
- PMCID: PMC2597642
- DOI: 10.1002/hep.22506
Molecular targeted therapies in hepatocellular carcinoma
Abstract
Hepatocellular carcinoma (HCC) is a complex and heterogeneous tumor with several genomic alterations. There is evidence of aberrant activation of several signaling cascades such as epidermal growth factor receptor (EGFR), Ras/extracellular signal-regulated kinase, phosphoinositol 3-kinase/mammalian target of rapamycin (mTOR), hepatocyte growth factor/mesenchymal-epithelial transition factor, Wnt, Hedgehog, and apoptotic signaling. Recently a multikinase inhibitor, sorafenib, has shown survival benefits in patients with advanced HCC. This advancement represents a breakthrough in the treatment of this complex disease and proves that molecular therapies can be effective in HCC. It is becoming apparent, however, that to overcome the complexity of genomic aberrations in HCC, combination therapies will be critical. Phase II studies have tested drugs blocking EGFR, vascular endothelial growth factor/platelet-derived growth factor receptor, and mTOR signaling. No relevant data has been produced so far in combination therapies. Future research is expected to identify new compounds to block important undruggable pathways, such as Wnt signaling, and to identify new oncogenes as targets for therapies through novel high-throughput technologies. Recent guidelines have established a new frame for the design of clinical trials in HCC. Randomized phase II trials with a time-to-progression endpoint are proposed as pivotal for capturing benefits from novel drugs. Survival remains the main endpoint to measure effectiveness in phase III studies. Patients assigned to the control arm should receive standard-of-care therapy, that is, chemoembolization for patients with intermediate-stage disease and sorafenib for patients with advanced-stage disease. Biomarkers and molecular imaging should be part of the trials, in order to optimize the enrichment of study populations and identify drug responders. Ultimately, a molecular classification of HCC based on genome-wide investigations and identification of patient subclasses according to drug responsiveness will lead to a more personalized medicine.
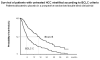
Figures




References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- El Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. - PubMed
-
- Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–54. - PubMed
-
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. - PubMed
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. The Lancet. 2003;362:1907–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
