The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial
- PMID: 18821708
- PMCID: PMC2836125
- DOI: 10.1002/art.23973
The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial
Abstract
Objective: Osteoarthritis (OA) of the knee causes significant morbidity and current medical treatment is limited to symptom relief, while therapies able to slow structural damage remain elusive. This study was undertaken to evaluate the effect of glucosamine and chondroitin sulfate (CS), alone or in combination, as well as celecoxib and placebo on progressive loss of joint space width (JSW) in patients with knee OA.
Methods: A 24-month, double-blind, placebo-controlled study, conducted at 9 sites in the United States as part of the Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT), enrolled 572 patients with knee OA who satisfied radiographic criteria (Kellgren/Lawrence [K/L] grade 2 or grade 3 changes and JSW of at least 2 mm at baseline). Patients with primarily lateral compartment narrowing at any time point were excluded. Patients who had been randomized to 1 of the 5 groups in the GAIT continued to receive glucosamine 500 mg 3 times daily, CS 400 mg 3 times daily, the combination of glucosamine and CS, celecoxib 200 mg daily, or placebo over 24 months. The minimum medial tibiofemoral JSW was measured at baseline, 12 months, and 24 months. The primary outcome measure was the mean change in JSW from baseline.
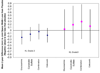
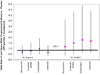
Results: The mean JSW loss at 2 years in knees with OA in the placebo group, adjusted for design and clinical factors, was 0.166 mm. No statistically significant difference in mean JSW loss was observed in any treatment group compared with the placebo group. Treatment effects on K/L grade 2 knees, but not on K/L grade 3 knees, showed a trend toward improvement relative to the placebo group. The power of the study was diminished by the limited sample size, variance of JSW measurement, and a smaller than expected loss in JSW.
Conclusion: At 2 years, no treatment achieved a predefined threshold of clinically important difference in JSW loss as compared with placebo. However, knees with K/L grade 2 radiographic OA appeared to have the greatest potential for modification by these treatments.
Trial registration: ClinicalTrials.gov NCT00032890.
Figures
Comment in
-
Methodology and statistical analysis in the Glucosamine/Chondroitin Arthritis Intervention Trial: comment on the article by Sawitzke et al.Arthritis Rheum. 2009 Nov;60(11):3514-5; author reply 3515-6. doi: 10.1002/art.24909. Arthritis Rheum. 2009. PMID: 19877051 No abstract available.
References
-
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. - PubMed
-
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

