Synthesis and biological evaluation of a phosphonate analog of the natural acetyl cholinesterase inhibitor cyclophostin
- PMID: 18821801
- PMCID: PMC2726048
- DOI: 10.1021/jo801453v
Synthesis and biological evaluation of a phosphonate analog of the natural acetyl cholinesterase inhibitor cyclophostin
Abstract
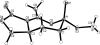
Two diastereomers of a phosphonate analog 6 of the AChE inhibitor cyclophostin were synthesized. The substitution reaction of phosphono allylic carbonate 10a with methyl acetoacetate gave the vinyl phosphonate 9a. Attempted hydrogenation/debenzylation gave an unexpected enolether lactone. Alternatively, selective hydrogenation, demethylation, cyclization and debenzylation gave the phosphonate analog of cyclophostin as a separable mixture of diastereomers 6. The trans phosphonate isomer was more active than the cis isomer against AChE from two sources.
Figures
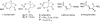


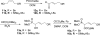

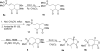

References
-
- Kurokawa T, Suzuki K, Hayaoka T, Nagakawa T, Izawa T, Kobayashi M, Harada M. J. Antibiot. 1993;46:1315–1318. - PubMed
-
- He WH. Phosphorus, Sulfur Silicon Relat. Elem. 2008;183:266.
-
- Neumann R, Peter HH. Experientia. 1987;43:1235.
-
- Garica-Carrasco M, Escárcega RO, Fuentes-Alexandro S, Riebeling C, Cervera R. Autoimmunity Reviews. 2007;6:373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

