Toll-like receptors in neurodegeneration
- PMID: 18822314
- PMCID: PMC2679904
- DOI: 10.1016/j.brainresrev.2008.09.001
Toll-like receptors in neurodegeneration
Abstract
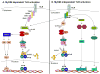
The key roles of toll-like receptors (TLRs) as mediators of the detection and responses of immune cells to invading pathogens are well known. There are at least 13 mammalian TLRs which are integral membrane proteins with a leucine-rich extracellular domain and a cytoplasmic domain similar to that of the interleukin-1 receptor which initiates downstream signaling through kinases to activate transcription factors such as AP-1 and NFkappaB. TLRs are activated in glial cells (microglia, astrocytes and oligodendrocytes) and lymphocytes that infiltrate the nervous system in response to inflammation caused by infectious agents, tissue injury or autoimmune conditions. By inducing the production of pro-inflammatory cytokines and cell adhesion molecules in immune cells, TLRs may indirectly damage neurons in conditions such as ischemic stroke and multiple sclerosis. Recent findings suggest that neurons also express a subset of TLRs and that their activation promotes neuronal degeneration in experimental models of stroke and Alzheimer's disease. TLRs may also play roles in regulating the processes of neurogenesis and neurite outgrowth, suggesting roles in neuronal plasticity. A better understanding of the molecular and cellular biology of TLRs in the normal and diseased nervous system, may lead to novel approaches for preventing neuronal degeneration and promoting recovery of function in an array of neurodegenerative conditions.
Figures

References
-
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. - PubMed
-
- Hashimoto C, et al. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. - PubMed
-
- Medzhitov R, et al. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
-
- Hoshino K, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. - PubMed
-
- Gerondakis S, et al. Regulating B-cell activation and survival in response to TLR signals. Immunol Cell Biol. 2007;85:471–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

