Cracking taste codes by tapping into sensory neuron impulse traffic
- PMID: 18824076
- PMCID: PMC2680288
- DOI: 10.1016/j.pneurobio.2008.09.003
Cracking taste codes by tapping into sensory neuron impulse traffic
Abstract
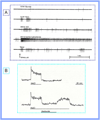
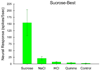
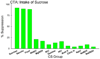
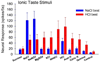
Insights into the biological basis for mammalian taste quality coding began with electrophysiological recordings from "taste" nerves and this technique continues to produce essential information today. Chorda tympani (geniculate ganglion) neurons, which are particularly involved in taste quality discrimination, are specialists or generalists. Specialists respond to stimuli characterized by a single taste quality as defined by behavioral cross-generalization in conditioned taste tests. Generalists respond to electrolytes that elicit multiple aversive qualities. Na(+)-salt (N) specialists in rodents and sweet-stimulus (S) specialists in multiple orders of mammals are well characterized. Specialists are associated with species' nutritional needs and their activation is known to be malleable by internal physiological conditions and contaminated external caloric sources. S specialists, associated with the heterodimeric G-protein coupled receptor T1R, and N specialists, associated with the epithelial sodium channel ENaC, are consistent with labeled line coding from taste bud to afferent neuron. Yet, S-specialist neurons and behavior are less specific than T1R2-3 in encompassing glutamate and E generalist neurons are much less specific than a candidate, PDK TRP channel, sour receptor in encompassing salts and bitter stimuli. Specialist labeled lines for nutrients and generalist patterns for aversive electrolytes may be transmitting taste information to the brain side by side. However, specific roles of generalists in taste quality coding may be resolved by selecting stimuli and stimulus levels found in natural situations. T2Rs, participating in reflexes via the glossopharynygeal nerve, became highly diversified in mammalian phylogenesis as they evolved to deal with dangerous substances within specific environmental niches. Establishing the information afferent neurons traffic to the brain about natural taste stimuli imbedded in dynamic complex mixtures will ultimately "crack taste codes."
Figures

Similar articles
-
Gustatory neuron types in rat geniculate ganglion.J Neurophysiol. 1999 Dec;82(6):2970-88. doi: 10.1152/jn.1999.82.6.2970. J Neurophysiol. 1999. PMID: 10601433
-
Taste-responsive neurons of the glossopharyngeal nerve of the rat.J Neurophysiol. 1991 Jun;65(6):1452-63. doi: 10.1152/jn.1991.65.6.1452. J Neurophysiol. 1991. PMID: 1875254
-
Response latency to lingual taste stimulation distinguishes neuron types within the geniculate ganglion.J Neurophysiol. 2010 Apr;103(4):1771-84. doi: 10.1152/jn.00785.2009. Epub 2010 Jan 27. J Neurophysiol. 2010. PMID: 20107132 Free PMC article.
-
Recognizing Taste: Coding Patterns Along the Neural Axis in Mammals.Chem Senses. 2019 Apr 15;44(4):237-247. doi: 10.1093/chemse/bjz013. Chem Senses. 2019. PMID: 30788507 Free PMC article. Review.
-
Neuron types, receptors, behavior, and taste quality.Physiol Behav. 2000 Apr 1-15;69(1-2):53-62. doi: 10.1016/s0031-9384(00)00188-8. Physiol Behav. 2000. PMID: 10854917 Review.
Cited by
-
Intravital microscopic interrogation of peripheral taste sensation.Sci Rep. 2015 Mar 2;5:8661. doi: 10.1038/srep08661. Sci Rep. 2015. PMID: 25726964 Free PMC article.
-
Taste coding after selective inhibition by chlorhexidine.Chem Senses. 2009 Oct;34(8):653-66. doi: 10.1093/chemse/bjp047. Epub 2009 Aug 24. Chem Senses. 2009. PMID: 19703921 Free PMC article.
-
Statistical analysis and decoding of neural activity in the rodent geniculate ganglion using a metric-based inference system.PLoS One. 2013 May 30;8(5):e65439. doi: 10.1371/journal.pone.0065439. Print 2013. PLoS One. 2013. PMID: 23738016 Free PMC article.
-
Amiloride-sensitive and amiloride-insensitive responses to NaCl + acid mixtures in hamster chorda tympani nerve.Chem Senses. 2012 Sep;37(7):603-12. doi: 10.1093/chemse/bjs042. Epub 2012 Mar 26. Chem Senses. 2012. PMID: 22451526 Free PMC article.
-
Time and intensity factors in identification of components of odor mixtures.Chem Senses. 2010 Nov;35(9):777-87. doi: 10.1093/chemse/bjq078. Epub 2010 Aug 18. Chem Senses. 2010. PMID: 20720093 Free PMC article.
References
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. - PubMed
-
- Anderson B, Landgren S, Olsson L, Zotterman Y. The sweet fibres of the dog. Acta Physiol Scand. 1950;21:105–119. - PubMed
-
- Axel R. Scents and sensibility: a molecular logic of olfactory perception (Nobel lecture) Angew Chem Int Ed. 2005;44:6111–6127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

