Bacteriophage lysins as effective antibacterials
- PMID: 18824123
- PMCID: PMC2597892
- DOI: 10.1016/j.mib.2008.09.012
Bacteriophage lysins as effective antibacterials
Abstract
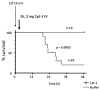
Lysins are highly evolved enzymes produced by bacteriophage (phage for short) to digest the bacterial cell wall for phage progeny release. In Gram-positive bacteria, small quantities of purified recombinant lysin added externally results in immediate lysis causing log-fold death of the target bacterium. Lysins have been used successfully in a variety of animal models to control pathogenic antibiotic resistant bacteria found on mucosal surfaces and infected tissues. The advantages over antibiotics are their specificity for the pathogen without disturbing the normal flora, the low chance of bacterial resistance to lysins, and their ability to kill colonizing pathogens on mucosal surfaces, a capacity previously unavailable. Thus, lysins may be a much needed anti-infective in an age of mounting antibiotic resistance.
Figures

References
-
- Wang I-N, Smith DL, Young R. Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol. 2000;54:799–825. - PubMed
-
- Young R, Wang I-N, Roof WD. Phages will out: strategies of host cell lysis. Trends in Micro. 2000;8:120–128. - PubMed
-
- Bernhardt TG, Wang IN, Struck DK, Young R. A protein antibiotic in the phage Q-beta virion: diversity in lysis targets. Science. 2001;292:2326–2329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

