L-Ascorbate biosynthesis in higher plants: the role of VTC2
- PMID: 18824398
- PMCID: PMC2583178
- DOI: 10.1016/j.tplants.2008.08.005
L-Ascorbate biosynthesis in higher plants: the role of VTC2
Abstract
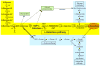
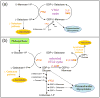
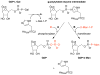
In the past year, the last missing enzyme of the L-galactose pathway, the linear form of which appears to represent the major biosynthetic route to L-ascorbate (vitamin C) in higher plants, has been identified as a GDP-L-galactose phosphorylase. This enzyme catalyzes the first committed step in the synthesis of that vital antioxidant and enzyme cofactor. Here, we discuss how GDP-L-galactose phosphorylase enzymes, encoded in Arabidopsis by the paralogous VTC2 and VTC5 genes, function in concert with the other enzymes of the L-galactose pathway to provide plants with the appropriate levels of L-ascorbate. We hypothesize that regulation of L-ascorbate biosynthesis might occur at more than one step and warrants further investigation to allow for the manipulation of vitamin C levels in plants.
Figures
References
-
- Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. - PubMed
-
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol. 2000;3:229–235. - PubMed
-
- Pignocchi C, Foyer CH. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr Opin Plant Biol. 2003;6:379–389. - PubMed
-
- de Pinto MC, et al. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J. 2006;48:784–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

