The 3' proximal translational enhancer of Turnip crinkle virus binds to 60S ribosomal subunits
- PMID: 18824512
- PMCID: PMC2578866
- DOI: 10.1261/rna.1227808
The 3' proximal translational enhancer of Turnip crinkle virus binds to 60S ribosomal subunits
Abstract
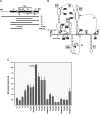
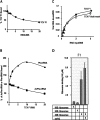
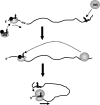
During cap-dependent translation of eukaryotic mRNAs, initiation factors interact with the 5' cap to attract ribosomes. When animal viruses translate in a cap-independent fashion, ribosomes assemble upstream of initiation codons at internal ribosome entry sites (IRES). In contrast, many plant viral genomes do not contain 5' ends with substantial IRES activity but instead have 3' translational enhancers that function by an unknown mechanism. A 393-nucleotide (nt) region that includes the entire 3' UTR of the Turnip crinkle virus (TCV) synergistically enhances translation of a reporter gene when associated with the TCV 5' UTR. The major enhancer activity was mapped to an internal region of approximately 140 nt that partially overlaps with a 100-nt structural domain previously predicted to adopt a form with some resemblance to a tRNA, according to a recent study by J.C. McCormack and colleagues. The T-shaped structure binds to 80S ribosomes and 60S ribosomal subunits, and binding is more efficient in the absence of surrounding sequences and in the presence of a pseudoknot that mimics the tRNA-acceptor stem. Untranslated TCV satellite RNA satC, which contains the TCV 3' end and 6-nt differences in the region corresponding to the T-shaped element, does not detectably bind to 80S ribosomes and is not predicted to form a comparable structure. Binding of the TCV T-shaped element by 80S ribosomes was unaffected by salt-washing, reduced in the presence of AcPhe-tRNA, which binds to the P-site, and enhanced binding of Phe-tRNA to the ribosome A site. Mutations that reduced translation in vivo had similar effects on ribosome binding in vitro. This strong correlation suggests that ribosome entry in the 3' UTR is a key function of the 3' translational enhancer of TCV and that the T-shaped element contains some tRNA-like properties.
Figures
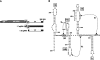



References
-
- Allen G.S., Frank J. Structural insights on the translation initiation complex: Ghosts of a universal initiation complex. Mol. Microbiol. 2007;63:941–950. - PubMed
-
- Barends S., Bink H.H., van den Worm S.H., Pleij C.W., Kraal B. Entrapping ribosomes for viral translation: tRNA mimicry as a molecular Trojan horse. Cell. 2003;112:123–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
