Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration
- PMID: 18824634
- PMCID: PMC2628327
- DOI: 10.1369/jhc.2008.952143
Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration
Abstract
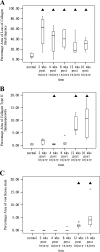
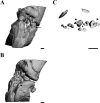
We report chondrocyte phenotype and ectopic ossification in a collagenase-induced patellar tendon injury model. Collagenase or saline was injected intratendinously in one limb. The patella tendon was harvested for assessment at different times. There was an increase in cellularity, vascularity, and loss of matrix organization with time after collagenase injection. The tendon did not heal histologically until week 32. Ectopic mineralization as indicated by von Kossa staining started from week 8. Tendon calcification was mediated by endochondral ossification, as shown by expression of type X collagen. viva CT imaging and polarization microscopy showed characteristic bony porous structures and collagen fiber arrangement, respectively, in the calcific regions. Marrow-like cells and blood vessels were observed inside calcific deposits. Chondrocyte-like cells as indicated by morphology, expression of type II collagen, and sox 9 were seen around and embedded inside the calcific deposits. Fibroblast-like cells expressed type II collagen and sox 9 at earlier times, suggesting that erroneous differentiation of healing tendon fibroblasts may account for failed healing and ossification in collagenase-induced tendon degeneration. Because this animal model replicates key histopathological changes in calcific tendinopathy, it can be used as a model for the study of its pathogenesis at the patellar tendon.
Figures


References
-
- Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ (2007) Rat supraspinatus tendon expresses cartilage makers with overuse. J Orthop Res 25:617–624 - PubMed
-
- Bi Y, Ehirchiou D, Kilts TM, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. (2007) Identification of tendon stem / progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13:1219–1227 - PubMed
-
- Chen YJ, Wang CJ, Yang KD, Kuo YR, Huang HC, Huang YT, Sun YC, et al. (2004) Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-beta1 and IGF-I expression. J Orthop Res 22:854–861 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

