Differential regulation of morphine antinociceptive effects by endogenous enkephalinergic system in the forebrain of mice
- PMID: 18826595
- PMCID: PMC2569012
- DOI: 10.1186/1744-8069-4-41
Differential regulation of morphine antinociceptive effects by endogenous enkephalinergic system in the forebrain of mice
Abstract
Background: Mice lacking the preproenkephalin (ppENK) gene are hyperalgesic and show more anxiety and aggression than wild-type (WT) mice. The marked behavioral changes in ppENK knock-out (KO) mice appeared to occur in supraspinal response to painful stimuli. However the functional role of enkephalins in the supraspinal nociceptive processing and their underlying mechanism is not clear. The aim of present study was to compare supraspinal nociceptive and morphine antinociceptive responses between WT and ppENK KO mice.
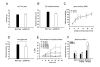
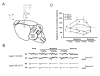
Results: The genotypes of bred KO mice were confirmed by PCR. Met-enkephalin immunoreactive neurons were labeled in the caudate-putamen, intermediated part of lateral septum, lateral globus pallidus, intermediated part of lateral septum, hypothalamus, and amygdala of WT mice. Met-enkephalin immunoreactive neurons were not found in the same brain areas in KO mice. Tail withdrawal and von Frey test results did not differ between WT and KO mice. KO mice had shorter latency to start paw licking than WT mice in the hot plate test. The maximal percent effect of morphine treatments (5 mg/kg and 10 mg/kg, i.p.) differed between WT and KO mice in hot plate test. The current source density (CSD) profiles evoked by peripheral noxious stimuli in the primary somatosenstory cortex (S1) and anterior cingulate cortex (ACC) were similar in WT and KO mice. After morphine injection, the amplitude of the laser-evoked sink currents was decreased in S1 while the amplitude of electrical-evoked sink currents was increased in the ACC. These differential morphine effects in S1 and ACC were enhanced in KO mice. Facilitation of synaptic currents in the ACC is mediated by GABA inhibitory interneurons in the local circuitry. Percent increases in opioid receptor binding in S1 and ACC were 5.1% and 5.8%, respectively.
Conclusion: The present results indicate that the endogenous enkephalin system is not involved in acute nociceptive transmission in the spinal cord, S1, and ACC. However, morphine preferentially suppressed supraspinal related nociceptive behavior in KO mice. This effect was reflected in the potentiated differential effects of morphine in the S1 and ACC in KO mice. This potentiation may be due to an up-regulation of opioid receptors. Thus these findings strongly suggest an antagonistic interaction between the endogenous enkephalinergic system and exogenous opioid analgesic actions in the supraspinal brain structures.
Figures





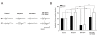

Similar articles
-
Neurofilament proteins and cAMP pathway in brains of mu-, delta- or kappa-opioid receptor gene knock-out mice: effects of chronic morphine administration.Neuropharmacology. 2004 Mar;46(4):519-30. doi: 10.1016/j.neuropharm.2003.10.006. Neuropharmacology. 2004. PMID: 14975676
-
Involvement of spinal Met-enkephalin in nicotine-induced antinociception in mice.Brain Res. 2008 Jan 16;1189:70-7. doi: 10.1016/j.brainres.2007.10.086. Epub 2007 Nov 7. Brain Res. 2008. PMID: 18048009
-
Peripheral antinociceptive effects of mu- and delta-opioid receptor agonists in NOS2 and NOS1 knockout mice during chronic inflammatory pain.Eur J Pharmacol. 2009 Jan 5;602(1):41-9. doi: 10.1016/j.ejphar.2008.11.019. Epub 2008 Nov 18. Eur J Pharmacol. 2009. PMID: 19041302
-
[The spinal enkephalinergic and serotoninergic systems in the control of transmission of nociceptive messages].J Pharmacol. 1985;16 Suppl 1:119-37. J Pharmacol. 1985. PMID: 2993751 Review. French.
-
[Enkephalin catabolism inhibitors and antalgics of the future: from preclinical research to clinical trials].Therapie. 1999 Jan-Feb;54(1):121-33. Therapie. 1999. PMID: 10216436 Review. French.
Cited by
-
Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site.Am J Pathol. 2010 Feb;176(2):786-99. doi: 10.2353/ajpath.2010.090457. Epub 2009 Dec 30. Am J Pathol. 2010. PMID: 20042674 Free PMC article.
-
Mu and delta opioid receptors play opposite nociceptive and behavioural roles on nerve-injured mice.Br J Pharmacol. 2020 Mar;177(5):1187-1205. doi: 10.1111/bph.14911. Epub 2020 Feb 10. Br J Pharmacol. 2020. PMID: 31655493 Free PMC article.
-
Ultrastructural relationship between the mu opioid receptor and its interacting protein, GPR177, in striatal neurons.Brain Res. 2010 Oct 28;1358:71-80. doi: 10.1016/j.brainres.2010.08.080. Epub 2010 Sep 21. Brain Res. 2010. PMID: 20813097 Free PMC article.
-
Modulation of brain electroencephalography oscillations by electroacupuncture in a rat model of postincisional pain.Evid Based Complement Alternat Med. 2013;2013:160357. doi: 10.1155/2013/160357. Epub 2013 Apr 28. Evid Based Complement Alternat Med. 2013. PMID: 23710210 Free PMC article.
-
Spontaneous Cingulate High-Current Spikes Signal Normal and Pathological Pain States.J Neurosci. 2019 Jun 26;39(26):5128-5142. doi: 10.1523/JNEUROSCI.2590-18.2019. Epub 2019 Apr 25. J Neurosci. 2019. PMID: 31023834 Free PMC article.
References
-
- Olson GA, Olson RD, Kastin AJ. Endogenous opiates: 1992. Peptides. 1993;14:1339–1378. - PubMed
-
- Olson GA, Olson RD, Kastin AJ. Endogenous opiates: 1996. Peptides. 1997;18:1651–1688. - PubMed
-
- Imura H, Kato Y, Nakai Y, Nakao K, Tanaka I, Jingami H, Koh T, Yoshimasa T, Tsukada T, Suda M, et al. Endogenous opioids and related peptides: from molecular biology to clinical medicine. The Sir Henry Dale lecture for 1985. J Endocrinol. 1985;107:147–157. - PubMed
-
- Noble F, Smadja C, Valverde O, Maldonado R, Coric P, Turcaud S, Fournie-Zaluski MC, Roques BP. Pain-suppressive effects on various nociceptive stimuli (thermal, chemical, electrical and inflammatory) of the first orally active enkephalin-metabolizing enzyme inhibitor RB 120. Pain. 1997;73:383–391. - PubMed
-
- al-Rodhan N, Chipkin R, Yaksh TL. The antinociceptive effects of SCH-3 a neutral endopeptidase (enkephalinase) inhibitor, microinjected into the periaqueductal, ventral medulla and amygdala. Brain Res. 2615;520:123–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

