Mutational optimization of the coelenterazine-dependent luciferase from Renilla
- PMID: 18826616
- PMCID: PMC2565673
- DOI: 10.1186/1746-4811-4-23
Mutational optimization of the coelenterazine-dependent luciferase from Renilla
Abstract
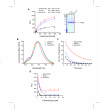
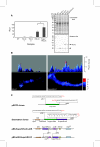
Renilla luciferase (RLUC) is a popular reporter enzyme for gene expression and biosensor applications, but it is an unstable enzyme whose catalytic mechanism remains to be elucidated. We titrated that one RLUC molecule can turn over about one hundred molecules of coelenterazine substrate. Mutagenesis of active site residue Pro220 extended the half-life of photon emission, yielding brighter luminescence in E. coli. Random mutagenesis uncovered two new mutations that stabilized and increased photon emission in vivo and in vitro, while ameliorating substrate inhibition. Further amended with a previously identified mutation, a new triple mutant showed a threefold improved kcat, as well as elevated luminescence in Arabidopsis. This advances the utility of RLUC as a reporter protein, biosensor, or resonance energy donor.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

