Activation of beta1,3-N-acetylglucosaminyltransferase-2 (beta3Gn-T2) by beta3Gn-T8. Possible involvement of beta3Gn-T8 in increasing poly-N-acetyllactosamine chains in differentiated HL-60 cells
- PMID: 18826941
- PMCID: PMC2662248
- DOI: 10.1074/jbc.M806933200
Activation of beta1,3-N-acetylglucosaminyltransferase-2 (beta3Gn-T2) by beta3Gn-T8. Possible involvement of beta3Gn-T8 in increasing poly-N-acetyllactosamine chains in differentiated HL-60 cells
Abstract
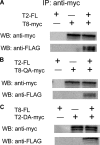
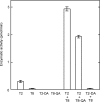
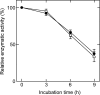
Enzymatic activities of some glycosyltransferases are markedly increased via complex formation with other transferases or cofactor proteins. We previously showed that beta1,3-N-acetylglucosaminyltransferase-2 (beta3Gn-T2) and beta3Gn-T8 can form a heterodimer in vitro and that the complex exhibits much higher enzymatic activity than either enzyme alone (Seko, A., and Yamashita, K. (2005) Glycobiology 15, 943-951). Here we examined this activation and the biological significance of complex formation in differentiated HL-60 cells. beta3Gn-T2 and -T8 were co-immunoprecipitated from the lysates of both-transfected COS-7 cells, indicating their association in vivo. We prepared inactive mutants of both enzymes by destroying the DXD motifs. The mixture of mutated beta3Gn-T2 and intact beta3Gn-T8 did not exhibit any activation, whereas the mixture of intact beta3Gn-T2 and mutated beta3Gn-T8 had increased activity, indicating the activation of beta3Gn-T2 via complex formation. Next, we compared expression levels of beta3Gn-T1-T8 in HL-60 cells and DMSO-treated differentiated HL-60 cells, which produce larger poly-N-acetyllactosamine chains. The expression level of beta3Gn-T8 in the differentiated cells was 2.6-fold higher than in the untreated cells. Overexpression of beta3Gn-T8, but not beta3Gn-T2, induced an increase in poly-N-acetyllactosamine chains in HL-60 cells. These results raise a possibility that up-regulation of beta3Gn-T8 in differentiated HL-60 cells increases poly-N-acetyllactosamine chains by activating intrinsic beta3Gn-T2.
Figures


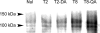

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

