TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton
- PMID: 18827015
- PMCID: PMC3539201
- DOI: 10.1242/jcs.029454
TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton
Abstract
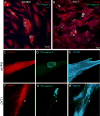
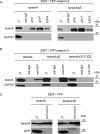
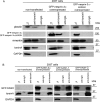
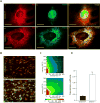
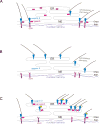
A specific mutation (DeltaE) in torsinA underlies most cases of the dominantly inherited movement disorder, early-onset torsion dystonia (DYT1). TorsinA, a member of the AAA+ ATPase superfamily, is located within the lumen of the nuclear envelope (NE) and endoplasmic reticulum (ER). We investigated an association between torsinA and nesprin-3, which spans the outer nuclear membrane (ONM) of the NE and links it to vimentin via plectin in fibroblasts. Mouse nesprin-3alpha co-immunoprecipitated with torsinA and this involved the C-terminal region of torsinA and the KASH domain of nesprin-3alpha. This association with human nesprin-3 appeared to be stronger for torsinADeltaE than for torsinA. TorsinA also associated with the KASH domains of nesprin-1 and -2 (SYNE1 and 2), which link to actin. In the absence of torsinA, in knockout mouse embryonic fibroblasts (MEFs), nesprin-3alpha was localized predominantly in the ER. Enrichment of yellow fluorescent protein (YFP)-nesprin-3 in the ER was also seen in the fibroblasts of DYT1 patients, with formation of YFP-positive globular structures enriched in torsinA, vimentin and actin. TorsinA-null MEFs had normal NE structure, but nuclear polarization and cell migration were delayed in a wound-healing assay, as compared with wild-type MEFs. These studies support a role for torsinA in dynamic interactions between the KASH domains of nesprins and their protein partners in the lumen of the NE, with torsinA influencing the localization of nesprins and associated cytoskeletal elements and affecting their role in nuclear and cell movement.
Figures





References
-
- Andra K, Kornacker I, Jorgl A, Zorer M, Spazierer D, Fuchs P, Fischer I, Wiche G. Plectin-isoform-specific rescue of hemidesmosomal defects in plectin (−/−) keratinocytes. J Invest Dermatol. 2003;120:189–197. - PubMed
-
- Asanuma K, Carbon-Correll M, Eidelberg D. Neuroimaging in human dystonia. J Med Invest. 2005;52:272–279. - PubMed
-
- Basham SE, Rose LS. The Caenorhabditis elegans polarity gene ooc-5 encodes a Torsin-related protein of the AAA ATPase superfamily. Development. 2001;128:4645–4656. - PubMed
-
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

