Distribution and evolution of circular miniproteins in flowering plants
- PMID: 18827180
- PMCID: PMC2570719
- DOI: 10.1105/tpc.108.062331
Distribution and evolution of circular miniproteins in flowering plants
Abstract
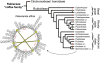
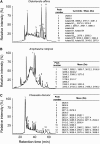
Cyclotides are disulfide-rich miniproteins with the unique structural features of a circular backbone and knotted arrangement of three conserved disulfide bonds. Cyclotides have been found only in two plant families: in every analyzed species of the violet family (Violaceae) and in few species of the coffee family (Rubiaceae). In this study, we analyzed >200 Rubiaceae species and confirmed the presence of cyclotides in 22 species. Additionally, we analyzed >140 species in related plant families to Rubiaceae and Violaceae and report the occurrence of cyclotides in the Apocynaceae. We further report new cyclotide sequences that provide insights into the mechanistic basis of cyclotide evolution. On the basis of the phylogeny of cyclotide-bearing plants and the analysis of cyclotide precursor gene sequences, we hypothesize that cyclotide evolution occurred independently in various plant families after the divergence of Asterids and Rosids ( approximately 125 million years ago). This is strongly supported by recent findings on the in planta biosynthesis of cyclotides, which involves the serendipitous recruitment of ubiquitous proteolytic enzymes for cyclization. We further predict that the number of cyclotides within the Rubiaceae may exceed tens of thousands, potentially making cyclotides one of the largest protein families in the plant kingdom.
Figures


References
-
- Backlund, M., Oxelman, B., and Bremer, B. (2000). Phylogenetic relationships within the Gentianales based on ndhF and rbcL sequences, with particular reference to the Loganiaceae. Am. J. Bot. 87 1029–1043. - PubMed
-
- Bokesch, H.R., Pannell, L.K., Cochran, P.K., Sowder, R.C., McKee, T.C., and Boyd, M.R. (2001). A novel anti-HIV macrocyclic peptide from Palicourea condensata. J. Nat. Prod. 64 249–250. - PubMed
-
- Bremer, B. (1996). Phylogenetic studies within Rubiaceae and relationships to other families based on molecular data. Opera Bot. Belg. 7 33–50.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

