Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1
- PMID: 18827182
- PMCID: PMC2570730
- DOI: 10.1105/tpc.108.058487
Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1
Abstract
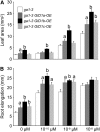
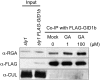
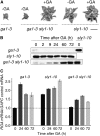
This article presents evidence that DELLA repression of gibberellin (GA) signaling is relieved both by proteolysis-dependent and -independent pathways in Arabidopsis thaliana. DELLA proteins are negative regulators of GA responses, including seed germination, stem elongation, and fertility. GA stimulates GA responses by causing DELLA repressor degradation via the ubiquitin-proteasome pathway. DELLA degradation requires GA biosynthesis, three functionally redundant GA receptors GIBBERELLIN INSENSITIVE DWARF1 (GID1a, b, and c), and the SLEEPY1 (SLY1) F-box subunit of an SCF E3 ubiquitin ligase. The sly1 mutants accumulate more DELLA proteins but display less severe dwarf and germination phenotypes than the GA biosynthesis mutant ga1-3 or the gid1abc triple mutant. Interestingly, GID1 overexpression rescued the sly1 dwarf and infertility phenotypes without decreasing the accumulation of the DELLA protein REPRESSOR OF ga1-3. GID1 rescue of sly1 mutants was dependent on the level of GID1 protein, GA, and the presence of a functional DELLA motif. Since DELLA shows increasing interaction with GID1 with increasing GA levels, it appears that GA-bound GID1 can block DELLA repressor activity by direct protein-protein interaction with the DELLA domain. Thus, a SLY1-independent mechanism for GA signaling may function without DELLA degradation.
Figures



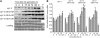

References
-
- Achard, P., Baghour, M., Chapple, A., Hedden, P., Van der Straeten, D., Genschik, P., Moritz, T., and Harberd, N.P. (2007). The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation ot floral meristem-identity genes. Proc. Natl. Acad. Sci. USA 104 6484–6489. - PMC - PubMed
-
- Achard, P., Cheng, H., De Grauwe, L., Decat, J., Schoutteten, H., Moritz, T., Van der Straeten, D., Peng, J.R., and Harberd, N.P. (2006). Integration of plant responses to environmentally activated phytohormonal signals. Science 311 91–94. - PubMed
-
- An, G., Ebert, R.R., Mitra, A., and Ha, S.B. (1988). Binary vectors. In Plant Molecular Biology Manual, S.B Gelvin and R.A. Schilpeoort, eds (Dordrecht, The Netherlands: Kluwer), pp. 1–19.
-
- Ariizumi, T., Kishitani, S., Inatsugi, R., Nishida, I., Murata, N., and Toriyama, K. (2002). An increase in unsaturation of fatty acids in phosphatidylglycerol from leaves improves the rates of photosynthesis and growth at low temperatures in transgenic rice seedlings. Plant Cell Physiol. 43 751–758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

