Clinical efficacy and predictive molecular markers of neoadjuvant gemcitabine and pemetrexed in resectable non-small cell lung cancer
- PMID: 18827606
- PMCID: PMC2639211
- DOI: 10.1097/JTO.0b013e3181874936
Clinical efficacy and predictive molecular markers of neoadjuvant gemcitabine and pemetrexed in resectable non-small cell lung cancer
Abstract
Background: A trial of neoadjuvant gemcitabine and pemetrexed (GP) chemotherapy in patients with resectable non-small cell lung cancer was conducted. The goal was to achieve a disease response rate of 50% and to determine if the expression levels of genes associated with GP metabolism are predictive of response.
Methods: Patients had staging with a computed tomography scan, whole body F-18 fluorodeoxyglucose positron emission tomography, and mediastinoscopy. Four biweekly cycles of GP were given. Patients were restaged, and those with resectable stage IB-III disease had thoracotomy. Fresh frozen tumor specimens were collected before and after chemotherapy and the mRNA levels of 14 target genes determined by real-time reverse transcription polymerase chain reaction.
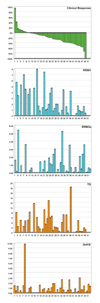
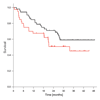
Results: Fifty-two patients started therapy. The radiographic disease response rate was 35% (95% confidence interval 21.7-49.6%), and the progression rate was 6%. Forty-six patients had a thoracotomy. The complete tumor resection rate was 77% (40/52). There were no perioperative deaths or deaths related to chemotherapy. Tumor response to chemotherapy was inversely correlated with the level of expression of RRM1 (p < 0.001; regulatory subunit of ribonucleotide reductase) and TS (p = 0.006; thymidylate synthase); i.e., the reduction in tumor size was greater in those with low levels of expression.
Conclusions: Neoadjuvant GP is well tolerated and produces an objective response rate of 35%. Tumoral RRM1 and TS mRNA levels are predictive of disease response and should be considered as parameters for treatment selection in future trials with this regimen.
Figures

References
-
- The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. - PubMed
-
- Winton TL, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin versus observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. - PubMed
-
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. - PubMed
-
- Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative Chemotherapy Followed by Surgery Compared With Primary Surgery in Resectable Stage I (Except T1N0), II, and IIIa Non-Small-Cell Lung Cancer. J Clin Oncol. 2002;20:247–253. - PubMed
-
- Scagliotti G. Preliminary results of Ch.E.S.T.: A phase III study of surgery alone or surgery plus preoperative gemcitabine-cisplatin in clinical early stages non-small cell lung cancer. Proc Am Soc Clin Oncol. 2005;23:626s. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

