Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model
- PMID: 18827803
- PMCID: PMC2834976
- DOI: 10.1038/mt.2008.220
Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model
Abstract
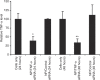
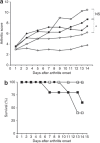
Secretion of tumor necrosis factor-alpha (TNF-alpha) by macrophages plays a predominant role in the development and progression of rheumatoid arthritis. We demonstrate that knockdown of TNF-alpha expression in systemic macrophages by intraperitoneal (i.p.) administration of chitosan/small interfering RNA (siRNA) nanoparticles in mice downregulates systemic and local inflammation. Chitosan nanoparticles containing an unmodified anti-TNF-alpha Dicer-substrate siRNA (DsiRNA) mediated TNF-alpha knockdown (approximately 66%) in primary peritoneal macrophages in vitro. The presence of Cy3-labeled nanoparticles within peritoneal macrophages and specific TNF-alpha knockdown (approximately 44%) with TNF-alpha siRNA after i.p. injection supports our therapeutic approach. Downregulation of TNF-alpha-induced inflammatory responses arrested joint swelling in collagen-induced arthritic (CIA) mice dosed i.p. with anti-TNF-alpha DsiRNA nanoparticles. The use of 2'-O-Me-modified DsiRNA resulted in the lowest arthritic scores and correlated with reduced type I interferon (IFN) activation in macrophages in vivo compared with unmodified DsiRNA. Histological analysis of joints revealed minimal cartilage destruction and inflammatory cell infiltration in anti-TNF-alpha-treated mice. The onset of arthritis could be delayed using a prophylactic dosing regime. This work demonstrates nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages as a method to reduce both local and systemic inflammation, thereby presenting a novel strategy for arthritis treatment.
Figures



References
-
- Arend WP. Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Rheum. 2001;45:101–106. - PubMed
-
- Williams RO, Marinova-Mutafchieva L, Feldmann M., and , Maini RN. Evaluation of TNF-α and IL-1 blockade in collagen-induced arthritis and comparison with combined anti-TNF-α/anti-CD4 therapy. J Immunol. 2000;165:7240–7245. - PubMed
-
- Taylor PC, Williams RO., and , Maini RN. Immunotherapy for rheumatoid arthritis. Curr Opin Immunol. 2001;13:611–616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

