The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation
- PMID: 18828769
- PMCID: PMC3087374
- DOI: 10.1111/j.1600-6143.2008.02377.x
The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation
Abstract
Regulatory T cells (Treg) are critical regulators of immune tolerance. Both IL-2 and CD28-CD80/CD86 signaling are critical for CD4(+)CD25(+)FOXP3(+) Treg survival in mice. Yet, both belatacept (a second-generation CTLA-4Ig) and basiliximab (an anti-CD25 monoclonal antibody) are among the arsenal of current immunotherapies being used in kidney transplant patients. In this study, we explored the direct effect of basiliximab and belatacept on the Tregs in peripheral blood both in the short term and long term and in kidney biopsies of patients with acute rejection. We report that the combined belatacept/basiliximab therapy has no long-term effect on circulating Tregs when compared to a calcineurin inhibitor (CNI)-treated group. Moreover, belatacept-treated patients had a significantly greater number of FOXP3(+) T cells in graft biopsies during acute rejection as compared to CNI-treated patients. Finally, it appears that the basiliximab caused a transient loss of both FOXP3(+) and FOXP3(-) CD25(+) T cells in the circulation in both treatment groups raising important questions about the use of this therapy in tolerance promoting therapeutic protocols.
Figures


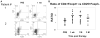

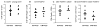




References
-
- Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. - PubMed
-
- Yamamoto AM, Chernajovsky Y, Lepault F, et al. The activity of immunoregulatory T cells mediating active tolerance is potentiated in nonobese diabetic mice by an IL-4-based retroviral gene therapy. J Immunol. 2001;166:4973–4980. - PubMed
-
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. - PubMed
-
- Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. - PubMed
-
- Curotto de Lafaille MA, Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

