Coxsackievirus B3 induction of NFAT: requirement for myocarditis susceptibility
- PMID: 18829062
- PMCID: PMC2590670
- DOI: 10.1016/j.virol.2008.08.020
Coxsackievirus B3 induction of NFAT: requirement for myocarditis susceptibility
Abstract
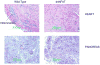
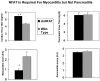
Ultraviolet (u.v.) inactivated coxsackievirus B3 (CVB3) induces rapid calcium flux in naïve BALB/c CD4+ T cells. CD4+ cells lacking decay accelerating factor (DAF-/-) show little calcium flux indicating that virus cross-linking of this virus receptor protein is necessary for calcium signaling in CVB3 infection. Interaction of CVB3 with CD4+ cells also activates NFAT DNA binding. To show that NFAT activation is crucial to CVB3 induced disease, wild-type mice and transgenic mice expressing dominant-negative NFAT (dnNFAT) mutant in T cells were infected and evaluated for myocarditis and pancreatitis 7 days later. Inhibition of NFAT in T cells prevented myocarditis but had no effect on pancreatitis. Virus titers in pancreas were equivalent in wild-type and dnNFAT animals but cardiac virus titers were increased in dnNFAT mice. Interferon-gamma (IFN gamma) expression was reduced in both CD4+ and V gamma 4+ T cells from dnNFAT mice compared to controls. FasL expression by V gamma 4+ cells was also suppressed. Inhibition of FasL expression by V gamma 4+ cells is consistent with myocarditis protection in dnNFAT mice.
Figures




Similar articles
-
Decay-accelerating factor (CD55) promotes CD1d expression and Vgamma4+ T-cell activation in coxsackievirus B3-induced myocarditis.Viral Immunol. 2006 Summer;19(2):156-66. doi: 10.1089/vim.2006.19.156. Viral Immunol. 2006. PMID: 16817758
-
IL-21R expression on CD8+ T cells promotes CD8+ T cell activation in coxsackievirus B3 induced myocarditis.Exp Mol Pathol. 2012 Jun;92(3):327-33. doi: 10.1016/j.yexmp.2012.03.009. Epub 2012 Mar 21. Exp Mol Pathol. 2012. PMID: 22465422 Free PMC article.
-
Expression of immunoregulatory cytokines by recombinant coxsackievirus B3 variants confers protection against virus-caused myocarditis.J Virol. 2001 Sep;75(17):8187-94. doi: 10.1128/jvi.75.17.8187-8194.2001. J Virol. 2001. PMID: 11483764 Free PMC article.
-
Inhibition of IL-2 inducible T-cell kinase alleviates T-cell activation and murine myocardial inflammation associated with CVB3 infection.Mol Immunol. 2014 May;59(1):30-8. doi: 10.1016/j.molimm.2013.12.004. Epub 2014 Jan 22. Mol Immunol. 2014. PMID: 24462896
-
Recombinant coxsackievirus vectors for prevention and therapy of virus-induced heart disease.Int J Med Microbiol. 2008 Jan;298(1-2):127-34. doi: 10.1016/j.ijmm.2007.08.010. Epub 2007 Sep 25. Int J Med Microbiol. 2008. PMID: 17897883 Review.
Cited by
-
Profiling Subcellular Protein Phosphatase Responses to Coxsackievirus B3 Infection of Cardiomyocytes.Mol Cell Proteomics. 2017 Apr;16(4 suppl 1):S244-S262. doi: 10.1074/mcp.O116.063487. Epub 2017 Feb 7. Mol Cell Proteomics. 2017. PMID: 28174228 Free PMC article.
-
Cross-talk between cd1d-restricted nkt cells and γδ cells in t regulatory cell response.Virol J. 2011 Jan 21;8:32. doi: 10.1186/1743-422X-8-32. Virol J. 2011. PMID: 21255407 Free PMC article. Review.
-
Cross-regulation of T regulatory-cell response after coxsackievirus B3 infection by NKT and γδ T cells in the mouse.Am J Pathol. 2013 Aug;183(2):441-9. doi: 10.1016/j.ajpath.2013.04.015. Epub 2013 Jun 5. Am J Pathol. 2013. PMID: 23746656 Free PMC article.
-
NFAT5 Restricts Bovine Herpesvirus 1 Productive Infection in MDBK Cell Cultures.Microbiol Spectr. 2023 Aug 17;11(4):e0011723. doi: 10.1128/spectrum.00117-23. Epub 2023 May 25. Microbiol Spectr. 2023. PMID: 37227295 Free PMC article.
-
Construction of miRNA-target networks using microRNA profiles of CVB3-infected HeLa cells.Sci Rep. 2019 Nov 29;9(1):17876. doi: 10.1038/s41598-019-54188-w. Sci Rep. 2019. PMID: 31784561 Free PMC article.
References
-
- Abreu MT, Arditi M. Innate immunity and toll-like receptors: clinical implications of basic science research. J Pediatr. 2004;144(4):421–9. - PubMed
-
- Bowles N, Richardson P, Olsen E, Archard L. Detection of coxsackie B virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;I:1120–1122. - PubMed
-
- Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42(3):466–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

