Explicit neural signals reflecting reward uncertainty
- PMID: 18829433
- PMCID: PMC2581779
- DOI: 10.1098/rstb.2008.0152
Explicit neural signals reflecting reward uncertainty
Abstract
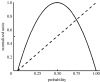
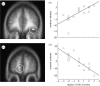
The acknowledged importance of uncertainty in economic decision making has stimulated the search for neural signals that could influence learning and inform decision mechanisms. Current views distinguish two forms of uncertainty, namely risk and ambiguity, depending on whether the probability distributions of outcomes are known or unknown. Behavioural neurophysiological studies on dopamine neurons revealed a risk signal, which covaried with the standard deviation or variance of the magnitude of juice rewards and occurred separately from reward value coding. Human imaging studies identified similarly distinct risk signals for monetary rewards in the striatum and orbitofrontal cortex (OFC), thus fulfilling a requirement for the mean variance approach of economic decision theory. The orbitofrontal risk signal covaried with individual risk attitudes, possibly explaining individual differences in risk perception and risky decision making. Ambiguous gambles with incomplete probabilistic information induced stronger brain signals than risky gambles in OFC and amygdala, suggesting that the brain's reward system signals the partial lack of information. The brain can use the uncertainty signals to assess the uncertainty of rewards, influence learning, modulate the value of uncertain rewards and make appropriate behavioural choices between only partly known options.
Figures

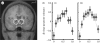
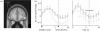
References
-
- Abel A.B. Asset prices under habit formation and catching up with the Joneses. Am. Econ. Rev. 1990;80:38–42.
-
- Basso M.A, Wurtz R.H. Modulation of neuronal activity by target uncertainty. Nature. 1997;398:66–69. doi:10.1038/37975 - DOI - PubMed
-
- Bechara A, Damasio A.R, Damasio H, Anderson S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi:10.1016/0010-0277(94)90018-3 - DOI - PubMed
-
- Bechara A, Damasio H, Damasio A.R. Emotion, decision-making and the orbitofrontal cortex. Cereb. Cortex. 2000;10:295–307. doi:10.1093/cercor/10.3.295 - DOI - PubMed
-
- Bolla K.I, Eldreth D.A, Matochik J.A, Cadet J.L. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi:10.1016/j.neuroimage.2005.02.012 - DOI - PubMed
