Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane
- PMID: 18829441
- PMCID: PMC2556629
- DOI: 10.1073/pnas.0804746105
Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane
Abstract
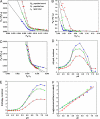
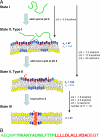
The pH low-insertion peptide (pHLIP) serves as a model system for peptide insertion and folding across a lipid bilayer. It has three general states: (I) soluble in water or (II) bound to the surface of a lipid bilayer as an unstructured monomer, and (III) inserted across the bilayer as a monomeric alpha-helix. We used fluorescence spectroscopy and isothermal titration calorimetry to study the interactions of pHLIP with a palmitoyloleoylphosphatidylcholine (POPC) lipid bilayer and to calculate the transition energies between states. We found that the Gibbs free energy of binding to a POPC surface at low pHLIP concentration (state I-state II transition) at 37 degrees C is approximately -7 kcal/mol near neutral pH and that the free energy of insertion and folding across a lipid bilayer at low pH (state II-state III transition) is nearly -2 kcal/mol. We discuss a number of related thermodynamic parameters from our measurements. Besides its fundamental interest as a model system for the study of membrane protein folding, pHLIP has utility as an agent to target diseased tissues and translocate molecules through the membrane into the cytoplasm of cells in environments with elevated levels of extracellular acidity, as in cancer and inflammation. The results give the amount of energy that might be used to move cargo molecules across a membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Comparison of lipid-dependent bilayer insertion of pHLIP and its P20G variant.Biochim Biophys Acta Biomembr. 2018 Feb;1860(2):534-543. doi: 10.1016/j.bbamem.2017.11.006. Epub 2017 Nov 11. Biochim Biophys Acta Biomembr. 2018. PMID: 29138065 Free PMC article.
-
A monomeric membrane peptide that lives in three worlds: in solution, attached to, and inserted across lipid bilayers.Biophys J. 2007 Oct 1;93(7):2363-72. doi: 10.1529/biophysj.107.109967. Epub 2007 Jun 8. Biophys J. 2007. PMID: 17557792 Free PMC article.
-
Thermodynamics of melittin binding to lipid bilayers. Aggregation and pore formation.Biochemistry. 2009 Mar 31;48(12):2586-96. doi: 10.1021/bi802127h. Biochemistry. 2009. PMID: 19173655
-
Membrane protein folding and stability: physical principles.Annu Rev Biophys Biomol Struct. 1999;28:319-65. doi: 10.1146/annurev.biophys.28.1.319. Annu Rev Biophys Biomol Struct. 1999. PMID: 10410805 Review.
-
Biophysics of pH-Driven Membrane Insertion: A Review of the pHLIP Peptide.J Phys Chem B. 2025 May 1;129(17):4123-4132. doi: 10.1021/acs.jpcb.5c00225. Epub 2025 Apr 18. J Phys Chem B. 2025. PMID: 40249795 Review.
Cited by
-
pH sensing at the intersection of tissue homeostasis and inflammation.Trends Immunol. 2023 Oct;44(10):807-825. doi: 10.1016/j.it.2023.08.008. Epub 2023 Sep 14. Trends Immunol. 2023. PMID: 37714775 Free PMC article. Review.
-
Kinetics of pHLIP peptide insertion into and exit from a membrane.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12095-12100. doi: 10.1073/pnas.1917857117. Epub 2020 May 14. Proc Natl Acad Sci U S A. 2020. PMID: 32409607 Free PMC article.
-
Stimuli-responsive nanoparticles for targeting the tumor microenvironment.J Control Release. 2015 Dec 10;219:205-214. doi: 10.1016/j.jconrel.2015.08.050. Epub 2015 Sep 1. J Control Release. 2015. PMID: 26341694 Free PMC article. Review.
-
Targeted acidosis mediated delivery of antigenic MHC-binding peptides.Front Immunol. 2024 Apr 11;15:1337973. doi: 10.3389/fimmu.2024.1337973. eCollection 2024. Front Immunol. 2024. PMID: 38665920 Free PMC article.
-
Optical molecular imaging for tumor detection and image-guided surgery.Biomaterials. 2018 Mar;157:62-75. doi: 10.1016/j.biomaterials.2017.12.002. Epub 2017 Dec 5. Biomaterials. 2018. PMID: 29245052 Free PMC article. Review.
References
-
- Engelman DM, et al. Membrane protein folding: beyond the two stage model. FEBS Lett. 2003;555:122–125. - PubMed
-
- Osborne AR, Rapoport TA. Protein translocation is mediated by oligomers of the SecY complex with one SecY copy forming the channel. Cell. 2007;129:97–110. - PubMed
-
- Borgese N, Brambillasca S, Colombo S. How tails guide tail-anchored proteins to their destinations. Curr Opin Cell Biol. 2007;19:368–375. - PubMed
-
- Engelman DM, Steitz TA. The spontaneous insertion of proteins into and across membranes: The helical hairpin hypothesis. Cell. 1981;23:411–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

