Conjugated equine estrogens and colorectal cancer incidence and survival: the Women's Health Initiative randomized clinical trial
- PMID: 18829444
- PMCID: PMC2937217
- DOI: 10.1158/1055-9965.EPI-08-0385
Conjugated equine estrogens and colorectal cancer incidence and survival: the Women's Health Initiative randomized clinical trial
Abstract
Background: In separate Women's Health Initiative randomized trials, combined hormone therapy with estrogen plus progestin reduced colorectal cancer incidence but estrogen alone in women with hysterectomy did not. We now analyze features of the colorectal cancers that developed and examine the survival of women following colorectal cancer diagnosis in the latter trial.
Participants and methods: 10,739 postmenopausal women who were 50 to 79 years of age and had undergone hysterectomy were randomized to conjugated equine estrogens (0.625 mg/d) or matching placebo. Colorectal cancer incidence was a component of the monitoring global index of the study but was not a primary study endpoint. Colorectal cancers were verified by central medical record and pathology report review. Bowel exam frequency was not protocol defined, but information on their use was collected.
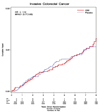
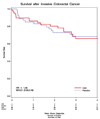
Results: After a median 7.1 years, there were 58 invasive colorectal cancers in the hormone group and 53 in the placebo group [hazard ratio, 1.12; 95% confidence interval (95% CI), 0.77-1.63]. Tumor size, stage, and grade were comparable in the two randomization groups. Bowel exam frequency was also comparable in the two groups. The cumulative mortality following colorectal cancer diagnosis among women in the conjugated equine estrogen group was 34% compared with 30% in the placebo group (hazard ratio, 1.34; 95% CI, 0.58-3.19).
Conclusions: In contrast to the preponderance of observational studies, conjugated equine estrogens in a randomized clinical trial did not reduce colorectal cancer incidence nor improve survival after diagnosis.
Figures
Similar articles
-
Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial.JAMA. 2011 Apr 6;305(13):1305-14. doi: 10.1001/jama.2011.382. JAMA. 2011. PMID: 21467283 Free PMC article. Clinical Trial.
-
Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial.J Natl Cancer Inst. 2010 Sep 22;102(18):1413-21. doi: 10.1093/jnci/djq285. Epub 2010 Aug 13. J Natl Cancer Inst. 2010. PMID: 20709992 Free PMC article. Clinical Trial.
-
Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study.JAMA. 2004 Jun 23;291(24):2947-58. doi: 10.1001/jama.291.24.2947. JAMA. 2004. PMID: 15213206 Clinical Trial.
-
The Women's Health Initiative Randomized Trials and Clinical Practice: A Review.JAMA. 2024 May 28;331(20):1748-1760. doi: 10.1001/jama.2024.6542. JAMA. 2024. PMID: 38691368 Review.
-
Estrogen therapy and breast cancer in randomized clinical trials: a narrative review.Menopause. 2022 Sep 1;29(9):1086-1092. doi: 10.1097/GME.0000000000002021. Epub 2022 Aug 16. Menopause. 2022. PMID: 35969882 Review.
Cited by
-
Increased risk of colon cancer in men in the pre-diabetes phase.PLoS One. 2013 Aug 2;8(8):e70426. doi: 10.1371/journal.pone.0070426. Print 2013. PLoS One. 2013. PMID: 23936428 Free PMC article.
-
Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause.Am J Epidemiol. 2009 Jul 1;170(1):12-23. doi: 10.1093/aje/kwp115. Epub 2009 May 25. Am J Epidemiol. 2009. PMID: 19468079 Free PMC article. Clinical Trial.
-
Postmenopausal hormone therapy and the risks of coronary heart disease, breast cancer, and stroke.Semin Reprod Med. 2014 Nov;32(6):419-25. doi: 10.1055/s-0034-1384624. Epub 2014 Oct 16. Semin Reprod Med. 2014. PMID: 25321418 Free PMC article. Review.
-
The Effect of Sex on the Azoxymethane/Dextran Sulfate Sodium-treated Mice Model of Colon Cancer.J Cancer Prev. 2016 Dec;21(4):271-278. doi: 10.15430/JCP.2016.21.4.271. Epub 2016 Dec 30. J Cancer Prev. 2016. PMID: 28053962 Free PMC article.
-
Colorectal Cancer Chemoprevention: A Dream Coming True?Int J Mol Sci. 2023 Apr 20;24(8):7597. doi: 10.3390/ijms24087597. Int J Mol Sci. 2023. PMID: 37108756 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2007. Ca Cancer J Clin. 2007;57:43–66. - PubMed
-
- Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340:101–107. - PubMed
-
- Steinbach G, Lynch PM, Phillips RKS, et al. The effect of celecoxib, a cyclooxygenase 2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. - PubMed
-
- Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. [Erratum, N Engl J Med 2003;348: 1939.] - PubMed
-
- Labayle D, Fischer D, Vielh P, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical