Sonic hedgehog promotes desmoplasia in pancreatic cancer
- PMID: 18829478
- PMCID: PMC2782957
- DOI: 10.1158/1078-0432.CCR-08-0291
Sonic hedgehog promotes desmoplasia in pancreatic cancer
Abstract
Purpose: We investigated the contribution of Sonic hedgehog (SHH) to pancreatic cancer progression.
Experimental design: We expressed SHH in a transformed primary ductal-derived epithelial cell line from the human pancreas, transformed hTert-HPNE (T-HPNE), and evaluated the effects on tumor growth. We also directly inhibited the activity of SHH in vivo by administering a blocking antibody to mice challenged orthotopically with the Capan-2 pancreatic cancer cell line, which is known to express SHH and form moderately differentiated tumors in nude mice.
Results: Our data provide evidence that expression of SHH influences tumor growth by contributing to the formation of desmoplasia in pancreatic cancer. We further show that SHH affects the differentiation and motility of human pancreatic stellate cells and fibroblasts.
Conclusions: These data suggest that SHH contributes to the formation of desmoplasia in pancreatic cancer, an important component of the tumor microenvironment.
Figures








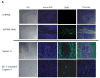

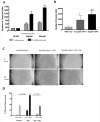
References
-
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. - PubMed
-
- Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. discussion 366-358. - PMC - PubMed
-
- Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. - PubMed
-
- Kayed H, Kleeff J, Osman T, Keleg S, Buchler MW, Friess H. Hedgehog signaling in the normal and diseased pancreas. Pancreas. 2006;32:119–129. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

