Dissociation of its opposing immunologic effects is critical for the optimization of antitumor CD8+ T-cell responses induced by interleukin 21
- PMID: 18829491
- PMCID: PMC2570356
- DOI: 10.1158/1078-0432.CCR-08-1146
Dissociation of its opposing immunologic effects is critical for the optimization of antitumor CD8+ T-cell responses induced by interleukin 21
Abstract
Purpose: Interleukin 21 (IL-21) is a promising new cytokine, which is undergoing clinical testing as an anticancer agent. Although IL-21 provides potent stimulation of CD8(+) T cells, it has also been suggested that IL-21 is immunosuppressive by counteracting the maturation of dendritic cells. The dissociation of these two opposing effects may enhance the utility of IL-21 as an immunotherapeutic. In this study, we used a cell-based artificial antigen-presenting cell (aAPC) lacking a functional IL-21 receptor (IL-21R) to investigate the immunostimulatory properties of IL-21.
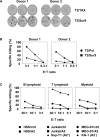
Experimental design: The immunosuppressive activity of IL-21 was studied using human IL-21R(+) dendritic cells. Antigen-specific CD8(+) T cells stimulated with human cell-based IL-21R(-)aAPC were used to isolate the T-cell immunostimulatory effects of IL-21. The functional outcomes, including phenotype, cytokine production, proliferation, and cytotoxicity were evaluated.
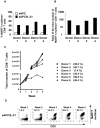
Results: IL-21 limits the immune response by maintaining immunologically immature dendritic cells. However, stimulation of CD8(+) T cells with IL-21R(-) aAPC, which secrete IL-21, results in significant expansion. Although priming in the presence of IL-21 temporarily modulated the T-cell phenotype, chronic stimulation abrogated these differences. Importantly, exposure to IL-21 during restimulation promoted the enrichment and expansion of antigen-specific CD8(+) T cells that maintained IL-2 secretion and gained enhanced IFN-gamma secretion. Tumor antigen-specific CTL generated in the presence of IL-21 recognized tumor cells efficiently, demonstrating potent effector functions.
Conclusions: IL-21 induces opposing effects on antigen-presenting cells and CD8(+) T cells. Strategic application of IL-21 is required to induce optimal clinical effects and may enable the generation of large numbers of highly avid tumor-specific CTL for adoptive immunotherapy.
Figures







Similar articles
-
Genetic modification of T cells with IL-21 enhances antigen presentation and generation of central memory tumor-specific cytotoxic T-lymphocytes.J Immunother. 2009 Sep;32(7):726-36. doi: 10.1097/CJI.0b013e3181ad4071. J Immunother. 2009. PMID: 19561536 Free PMC article.
-
Long-lived antitumor CD8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell.Clin Cancer Res. 2007 Mar 15;13(6):1857-67. doi: 10.1158/1078-0432.CCR-06-1905. Clin Cancer Res. 2007. PMID: 17363542
-
Ex vivo expansion of human CD8+ T cells using autologous CD4+ T cell help.PLoS One. 2012;7(1):e30229. doi: 10.1371/journal.pone.0030229. Epub 2012 Jan 12. PLoS One. 2012. PMID: 22279573 Free PMC article.
-
Multiple effects of immunostimulatory DNA on T cells and the role of type I interferons.Springer Semin Immunopathol. 2000;22(1-2):77-84. doi: 10.1007/s002810000028. Springer Semin Immunopathol. 2000. PMID: 10944802 Review.
-
The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer.Curr Opin Immunol. 2008 Jun;20(3):295-301. doi: 10.1016/j.coi.2008.02.004. Epub 2008 Jun 12. Curr Opin Immunol. 2008. PMID: 18554883 Free PMC article. Review.
Cited by
-
IL-21-treated naive CD45RA+ CD8+ T cells represent a reliable source for producing leukemia-reactive cytotoxic T lymphocytes with high proliferative potential and early differentiation phenotype.Cancer Immunol Immunother. 2011 Feb;60(2):235-48. doi: 10.1007/s00262-010-0936-8. Epub 2010 Nov 3. Cancer Immunol Immunother. 2011. PMID: 21046101 Free PMC article.
-
Human cell-based artificial antigen-presenting cells for cancer immunotherapy.Immunol Rev. 2014 Jan;257(1):191-209. doi: 10.1111/imr.12129. Immunol Rev. 2014. PMID: 24329798 Free PMC article. Review.
-
IL-21 can supplement suboptimal Lck-independent MAPK activation in a STAT-3-dependent manner in human CD8(+) T cells.J Immunol. 2012 Feb 15;188(4):1609-19. doi: 10.4049/jimmunol.1003446. Epub 2012 Jan 11. J Immunol. 2012. PMID: 22238455 Free PMC article.
-
An integrated disease/pharmacokinetic/pharmacodynamic model suggests improved interleukin-21 regimens validated prospectively for mouse solid cancers.PLoS Comput Biol. 2011 Sep;7(9):e1002206. doi: 10.1371/journal.pcbi.1002206. Epub 2011 Sep 29. PLoS Comput Biol. 2011. PMID: 22022259 Free PMC article.
-
A panel of human cell-based artificial APC enables the expansion of long-lived antigen-specific CD4+ T cells restricted by prevalent HLA-DR alleles.Int Immunol. 2010 Nov;22(11):863-73. doi: 10.1093/intimm/dxq440. Epub 2010 Nov 8. Int Immunol. 2010. PMID: 21059769 Free PMC article.
References
-
- Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annual review of immunology. 2007;25:243–65. - PubMed
-
- Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nature reviews. 2005;5:296–306. - PubMed
-
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nature medicine. 2004;10:475–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

