Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers
- PMID: 18829512
- PMCID: PMC2582814
- DOI: 10.1158/1078-0432.CCR-08-1247
Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers
Abstract
Purpose: 5-Azacytidine (5-AZA) is a DNA-hypomethylating agent. Valproic acid is a histone deacetylase inhibitor. Combining hypomethylating agents and histone deacetylase inhibitors produces synergistic anticancer activity in vitro and in vivo. On the basis of this evidence, we conducted a phase I study of the combination of 5-AZA and valproic acid in patients with advanced cancers.
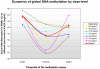
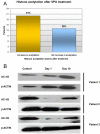
Experimental design: 5-AZA was administered s.c. daily for 10 days. Valproic acid was given orally daily with a goal to titrate to plasma levels of 75 to 100 mug/mL (therapeutic for seizures). Cycles were 28 days long. 5-AZA was started at 20 mg/m(2) and escalated using an adaptive algorithm based on the toxicity profile in the prior cohort (6 + 6 design). Peripheral blood mononuclear cell global DNA methylation and histone H3 acetylation were estimated with the long interspersed nucleotide elements pyrosequencing assay and Western blots, respectively, on days 1 and 10 of each cycle when patients agreed to provide them.
Results: Fifty-five patients were enrolled. Median age was 60 years (range, 12-77 years). The maximum tolerated dose was 75 mg/m(2) of 5-AZA in combination with valproic acid. Dose-limiting toxicities were neutropenic fever and thrombocytopenia, which occurred at a dose of 94 mg/m(2) of 5-AZA. Stable disease lasting 4 to 12 months (median, 6 months) was observed in 14 patients (25%). A significant decrease in global DNA methylation and induction of histone acetylation were observed.
Conclusion: The combination of 5-AZA and valproic acid is safe at doses up to 75 mg/m(2) for 5-AZA in patients with advanced malignancies.
Figures
References
-
- Goffin J, Eisenhauer E. DNA methyltransferase inhibitors-state of the art. Ann Oncol. 2002;13:1699–716. - PubMed
-
- Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23:3971–93. - PubMed
-
- Toyota M, Issa JP. Epigenetic changes in solid and hematopoietic tumors. Semin Oncol. 2005;32:521–30. - PubMed
-
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. - PubMed
-
- Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

