Excretion of human immunodeficiency virus type 1 through polarized epithelium by immunoglobulin A
- PMID: 18829757
- PMCID: PMC2583660
- DOI: 10.1128/JVI.01111-08
Excretion of human immunodeficiency virus type 1 through polarized epithelium by immunoglobulin A
Abstract
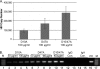
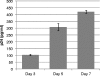
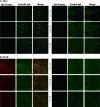
Human immunodeficiency virus (HIV) is transmitted primarily sexually across mucosal surfaces. After infection, HIV propagates initially in the lamina propria below the polarized epithelium and causes extensive destruction of mucosal T cells. Immunoglobulin A (IgA) antibodies, produced in the lamina propria and then transcytosed across the mucosal epithelium into the lumen, can be the first line of immune defense against HIV. Here, we used IgA monoclonal antibodies against HIV envelope proteins to investigate the abilities of polarized primate and human epithelial cells to excrete HIV virions from the basolateral to the apical surface via polymeric Ig receptor (pIgR)-mediated binding and the internalization of HIV-IgA immune complexes. African green monkey kidney cells expressing pIgR demonstrated HIV excretion that was dependent on the IgA concentration and the exposure time. Matched IgG antibodies with the same variable regions as the IgA antibodies and IgA antibodies to non-HIV antigens had no HIV excretory function. A mixture of two IgA anti-bodies against gp120 and gp41 showed a synergistic increase in the level of HIV excreted. The capacity for HIV excretion correlated with the ability of IgA antibodies to bind HIV and of the resulting immune complexes to bind pIgR. Consistent with the epithelial transcytosis of HIV-IgA immune complexes, the colocalization of HIV proteins and HIV-specific IgA was detected intracellularly by confocal microscopy. Our results suggest the potential of IgA antibodies to excrete HIV from mucosal lamina propria, thereby decreasing the viral burden, access to susceptible cells, and the chronic activation of the immune system.
Figures
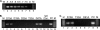
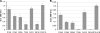



References
-
- Alfsen, A., P. Iniguez, E. Bouguyon, and M. Bomsel. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 1666257-6265. - PubMed
-
- Allaway, G. P., A. M. Ryder, G. A. Beaudry, and P. J. Maddon. 1993. Synergistic inhibition of HIV-1 envelope-mediated cell fusion by CD4-based molecules in combination with antibodies to gp120 or gp41. AIDS Res. Hum. Retrovir. 9581-587. - PubMed
-
- Ambrose, Z., K. Larsen, J. Thompson, Y. Stevens, E. Finn, S. L. Hu, and M. L. Bosch. 2001. Evidence for early local viral replication and local production of antiviral immunity upon mucosal simian-human immunodeficiency virus SHIV89.6 infection in Macaca nemestrina. J. Virol. 758589-8596. - PMC - PubMed
-
- Amerongen, H. M., R. Weltzin, C. M. Farnet, P. Michetti, W. A. Haseltine, and M. R. Neutra. 1991. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J. Acquir. Immune Defic. Syndr. 4760-765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

