Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells
- PMID: 18829761
- PMCID: PMC2593360
- DOI: 10.1128/JVI.01181-08
Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells
Abstract
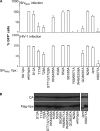
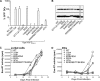
Human immunodeficiency virus type 2 (HIV-2)/simian immunodeficiency virus SIV(SM) Vpx is incorporated into virion particles and is thus present during the early steps of infection, when it has been reported to influence the nuclear import of viral DNA. We recently reported that Vpx promoted the accumulation of full-length viral DNA following the infection of human monocyte-derived dendritic cells (DCs). This positive effect was exerted following the infection of DCs with cognate viruses and with retroviruses as divergent as HIV-1, feline immunodeficiency virus, and even murine leukemia virus, leading us to suggest that Vpx counteracted an antiviral restriction present in DCs. Here, we show that Vpx is required, albeit to a different extent, for the infection of all myeloid but not of lymphoid cells, including monocytes, macrophages, and monocytoid THP-1 cells that had been induced to differentiate with phorbol esters. The intracellular localization of Vpx was highly heterogeneous and cell type dependent, since Vpx localized differently in HeLa cells and DCs. Despite these differences, no clear correlation between the functionality of Vpx and its intracellular localization could be drawn. As a first insight into its function, we determined that SIV(SM)/HIV-2 and SIV(RCM) Vpx proteins interact with the DCAF1 adaptor of the Cul4-based E3 ubiquitin ligase complex recently described to associate with HIV-1 Vpr and HIV-2 Vpx. However, the functionality of Vpx proteins in the infection of DCs did not strictly correlate with DCAF1 binding, and knockdown experiments failed to reveal a functional role for this association in differentiated THP-1 cells. Lastly, when transferred in the context of a replication-competent viral clone, Vpx was required for replication in DCs.
Figures

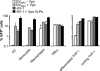



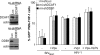
References
-
- Akari, H., J. Sakuragi, Y. Takebe, K. Tomonaga, M. Kawamura, M. Fukasawa, T. Miura, T. Shinjo, and M. Hayami. 1992. Biological characterization of human immunodeficiency virus type 1 and type 2 mutants in human peripheral blood mononuclear cells. Arch. Virol. 123157-167. - PubMed
-
- Belshan, M., L. A. Mahnke, and L. Ratner. 2006. Conserved amino acids of the human immunodeficiency virus type 2 Vpx nuclear localization signal are critical for nuclear targeting of the viral preintegration complex in non-dividing cells. Virology 346118-126. - PubMed
-
- Belshan, M., and L. Ratner. 2003. Identification of the nuclear localization signal of human immunodeficiency virus type 2 Vpx. Virology 3117-15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

