Development of theta rhythmicity in entorhinal stellate cells of the juvenile rat
- PMID: 18829850
- PMCID: PMC2604849
- DOI: 10.1152/jn.90424.2008
Development of theta rhythmicity in entorhinal stellate cells of the juvenile rat
Abstract
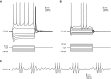
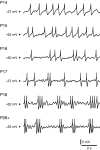
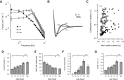
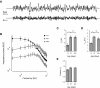
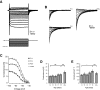
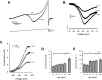
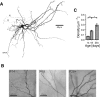
Mature stellate cells of the rat medial entorhinal cortex (EC), layer II, exhibit subthreshold membrane potential oscillations (MPOs) at theta frequencies (4-12 Hz) in vitro. We find that MPOs appear between postnatal days 14 (P14) and 18 (P18) but show little further change by day 28+ (P28-P32). To identify the factors responsible, we examined the electrical responses of developing stellate cells, paying attention to two currents thought necessary for mature oscillation: the h current I(h), which provides the slow rectification required for resonance; and a persistent sodium current I(NaP), which provides amplification of resonance. Responses to injected current revealed that P14 cells were often nonresonant with a relatively high resistance. Densities of I(h) and I(NaP) both rose by about 50% from P14 to P18. However, I(h) levels fell to intermediate values by P28+. Given the nonrobust trend in I(h) expression and a previously demonstrated potency of even low levels of I(h) to sustain oscillation, we propose that resonance and MPOs are limited at P14 more by low levels of I(NaP) than of I(h). The relative importance of I(NaP) for the development of MPOs is supported by simulations of a conductance-based model, which also suggest that general shunt conductance may influence the precise age when MPOs appear. In addition to our physiological study, we analyzed spine densities at P14, P18, and P28+ and found a vigorous synaptogenesis across the whole period. Our data predict that functions that rely on theta rhythmicity in the hippocampal network are limited until at least P18.
Figures
Similar articles
-
Membrane potential-dependent integration of synaptic inputs in entorhinal stellate neurons.Hippocampus. 2014 Dec;24(12):1493-505. doi: 10.1002/hipo.22329. Epub 2014 Jul 25. Hippocampus. 2014. PMID: 25044927 Free PMC article.
-
Dynamics of rat entorhinal cortex layer II and III cells: characteristics of membrane potential resonance at rest predict oscillation properties near threshold.J Physiol. 2004 Oct 1;560(Pt 1):89-110. doi: 10.1113/jphysiol.2004.069930. Epub 2004 Jul 22. J Physiol. 2004. PMID: 15272028 Free PMC article.
-
Biophysical properties and slow voltage-dependent inactivation of a sustained sodium current in entorhinal cortex layer-II principal neurons: a whole-cell and single-channel study.J Gen Physiol. 1999 Oct;114(4):491-509. doi: 10.1085/jgp.114.4.491. J Gen Physiol. 1999. PMID: 10498669 Free PMC article.
-
Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons.J Neurophysiol. 2000 May;83(5):2562-79. doi: 10.1152/jn.2000.83.5.2562. J Neurophysiol. 2000. PMID: 10805658
-
Characterization of voltage-gated K+ currents contributing to subthreshold membrane potential oscillations in hippocampal CA1 interneurons.J Neurophysiol. 2010 Jun;103(6):3472-89. doi: 10.1152/jn.00848.2009. Epub 2010 Apr 14. J Neurophysiol. 2010. PMID: 20393060
Cited by
-
Development of network oscillations through adolescence in male and female rats.Front Cell Neurosci. 2023 May 5;17:1135154. doi: 10.3389/fncel.2023.1135154. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37213214 Free PMC article.
-
Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala.J Physiol. 2012 Oct 1;590(19):4819-38. doi: 10.1113/jphysiol.2012.237453. Epub 2012 Jul 30. J Physiol. 2012. PMID: 22848043 Free PMC article.
-
Perinatal development of central vestibular neurons in mice.Front Neurosci. 2022 Sep 1;16:935166. doi: 10.3389/fnins.2022.935166. eCollection 2022. Front Neurosci. 2022. PMID: 36117641 Free PMC article.
-
The past, present, and future of real-time control in cellular electrophysiology.IEEE Trans Biomed Eng. 2014 May;61(5):1448-56. doi: 10.1109/TBME.2014.2314619. Epub 2014 Apr 1. IEEE Trans Biomed Eng. 2014. PMID: 24710815 Free PMC article. Review.
-
Membrane potential-dependent integration of synaptic inputs in entorhinal stellate neurons.Hippocampus. 2014 Dec;24(12):1493-505. doi: 10.1002/hipo.22329. Epub 2014 Jul 25. Hippocampus. 2014. PMID: 25044927 Free PMC article.
References
-
- Alonso A, García-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Exp Brain Res 67: 493–501, 1987. - PubMed
-
- Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol 70: 128–143, 1993. - PubMed
-
- Alonso A, Llinás RR. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342: 175–177, 1989. - PubMed
-
- Ben-Ari Y Developing networks play a similar melody. Trends Neurosci 24: 353–359, 2001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

