Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap
- PMID: 18829973
- PMCID: PMC2578837
- DOI: 10.1523/JNEUROSCI.2432-08.2008
Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap
Abstract
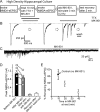
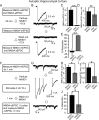
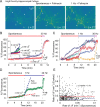
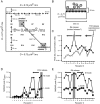
In a synapse, spontaneous and action-potential-driven neurotransmitter release is assumed to activate the same set of postsynaptic receptors. Here, we tested this assumption using (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate (MK-801), a well characterized use-dependent blocker of NMDA receptors. NMDA-receptor-mediated spontaneous miniature EPSCs (NMDA-mEPSCs) were substantially decreased by MK-801 within 2 min in a use-dependent manner. In contrast, MK-801 application at rest for 10 min did not significantly impair the subsequent NMDA-receptor-mediated evoked EPSCs (NMDA-eEPSCs). Brief stimulation in the presence of MK-801 significantly depressed evoked NMDA-eEPSCs but only mildly affected the spontaneous NMDA-mEPSCs detected on the same cell. Optical imaging of synaptic vesicle fusion showed that spontaneous and evoked release could occur at the same synapse albeit without correlation between their kinetics. In addition, modeling glutamate diffusion and NMDA receptor activation revealed that postsynaptic densities larger than approximately 0.2 microm(2) can accommodate two populations of NMDA receptors with nonoverlapping responsiveness. Collectively, these results support the premise that spontaneous and evoked neurotransmissions activate distinct sets of NMDA receptors and signal independently to the postsynaptic side.
Figures




References
-
- Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. - PubMed
-
- Colméus C, Gomez S, Molgó J, Thesleff S. Discrepancies between spontaneous and evoked synaptic potentials at normal, regenerating and botulinum toxin poisoned mammalian neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1982;215:63–74. - PubMed
-
- Courant R, Friedrichs K, Lewy H. Über die partiellen differenzengleichungen der mathematischen Physik. Mathematische Annalen. 1928;100:32–74.
-
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004 re16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
