Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis
- PMID: 18829990
- PMCID: PMC2584136
- DOI: 10.2337/db08-0805
Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis
Erratum in
- Diabetes. 2009 Mar;58(3):770
Abstract
Objective: Many of the effects of angiotensin (Ang) II are mediated through specific plasma membrane receptors. However, Ang II also elicits biological effects from the interior of the cell (intracrine), some of which are not inhibited by Ang receptor blockers (ARBs). Recent in vitro studies have identified high glucose as a potent stimulus for the intracellular synthesis of Ang II, the production of which is mainly chymase dependent. In the present study, we determined whether hyperglycemia activates the cardiac intracellular renin-Ang system (RAS) in vivo and whether ARBs, ACE, or renin inhibitors block synthesis and effects of intracellular Ang II (iAng II).
Research design and methods: Diabetes was induced in adult male rats by streptozotocin. Diabetic rats were treated with insulin, candesartan (ARB), benazepril (ACE inhibitor), or aliskiren (renin inhibitor).
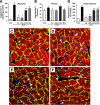
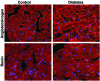
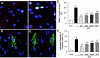
Results: One week of diabetes significantly increased iAng II levels in cardiac myocytes, which were not normalized by candesartan, suggesting that Ang II was synthesized intracellularly, not internalized through AT(1) receptor. Increased intracellular levels of Ang II, angiotensinogen, and renin were observed by confocal microscopy. iAng II synthesis was blocked by aliskiren but not by benazepril. Diabetes-induced superoxide production and cardiac fibrosis were partially inhibited by candesartan and benazepril, whereas aliskiren produced complete inhibition. Myocyte apoptosis was partially inhibited by all three agents.
Conclusions: Diabetes activates the cardiac intracellular RAS, which increases oxidative stress and cardiac fibrosis. Renin inhibition has a more pronounced effect than ARBs and ACE inhibitors on these diabetes complications and may be clinically more efficacious.
Figures



Similar articles
-
Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production.Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1675-84. doi: 10.1152/ajpheart.91493.2007. Epub 2008 Feb 22. Am J Physiol Heart Circ Physiol. 2008. PMID: 18296558
-
Direct renin inhibition prevents cardiac dysfunction in a diabetic mouse model: comparison with an angiotensin receptor antagonist and angiotensin-converting enzyme inhibitor.Clin Sci (Lond). 2013 Apr;124(8):529-41. doi: 10.1042/CS20120448. Clin Sci (Lond). 2013. PMID: 23116220 Free PMC article.
-
Angiotensin type 1a receptor-deficient mice develop diabetes-induced cardiac dysfunction, which is prevented by renin-angiotensin system inhibitors.Cardiovasc Diabetol. 2013 Nov 12;12:169. doi: 10.1186/1475-2840-12-169. Cardiovasc Diabetol. 2013. PMID: 24215514 Free PMC article.
-
Angiotensin II reactivation and aldosterone escape phenomena in renin-angiotensin-aldosterone system blockade: is oral renin inhibition the solution?Expert Opin Pharmacother. 2007 Apr;8(5):529-35. doi: 10.1517/14656566.8.5.529. Expert Opin Pharmacother. 2007. PMID: 17376010 Review.
-
Novel Cardiac Intracrine Mechanisms Based on Ang-(1-12)/Chymase Axis Require a Revision of Therapeutic Approaches in Human Heart Disease.Curr Hypertens Rep. 2017 Feb;19(2):16. doi: 10.1007/s11906-017-0708-3. Curr Hypertens Rep. 2017. PMID: 28233239 Free PMC article. Review.
Cited by
-
Saccharomyces boulardii modulates oxidative stress and renin angiotensin system attenuating diabetes-induced liver injury in mice.Sci Rep. 2021 Apr 28;11(1):9189. doi: 10.1038/s41598-021-88497-w. Sci Rep. 2021. PMID: 33911129 Free PMC article.
-
Evidence for a functional intracellular angiotensin system in the proximal tubule of the kidney.Am J Physiol Regul Integr Comp Physiol. 2012 Mar 1;302(5):R494-509. doi: 10.1152/ajpregu.00487.2011. Epub 2011 Dec 14. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22170616 Free PMC article. Review.
-
Maternal high-salt intake during pregnancy reprogrammed renin-angiotensin system-mediated cardiomyocyte apoptosis in the adult offspring heart.Reprod Sci. 2014 Jan;21(1):52-62. doi: 10.1177/1933719113488447. Epub 2013 May 20. Reprod Sci. 2014. PMID: 23690339 Free PMC article.
-
Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy.PLoS One. 2013 Apr 11;8(4):e60931. doi: 10.1371/journal.pone.0060931. Print 2013. PLoS One. 2013. PMID: 23593350 Free PMC article.
-
Network modeling reveals steps in angiotensin peptide processing.Hypertension. 2013 Mar;61(3):690-700. doi: 10.1161/HYPERTENSIONAHA.111.00318. Epub 2013 Jan 2. Hypertension. 2013. PMID: 23283355 Free PMC article.
References
-
- Paul M, Poyan Mehr A, Kreutz R: Physiology of local renin-angiotensin systems. Physiol Rev 86 :747 –803,2006 - PubMed
-
- Connelly KA, Boyle AJ, Kelly DJ: Angiotensin II and the cardiac complications of diabetes mellitus. Curr Pharm Des 13 :2721 –2729,2007 - PubMed
-
- Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD: Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int 50 :1897 –1903,1996 - PubMed
-
- Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M: Increased plasma inactive renin in diabetes mellitus: a marker of microvascular complications. N Engl J Med 312 :1412 –1417,1985 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

