Cerebrospinal fluid dendritic cells infiltrate the brain parenchyma and target the cervical lymph nodes under neuroinflammatory conditions
- PMID: 18830405
- PMCID: PMC2552991
- DOI: 10.1371/journal.pone.0003321
Cerebrospinal fluid dendritic cells infiltrate the brain parenchyma and target the cervical lymph nodes under neuroinflammatory conditions
Abstract
Background: In many neuroinflammatory diseases, dendritic cells (DCs) accumulate in several compartments of the central nervous system (CNS), including the cerebrospinal fluid (CSF). Myeloid DCs invading the inflamed CNS are thus thought to play a major role in the initiation and perpetuation of CNS-targeted autoimmune responses. We previously reported that, in normal rats, DCs injected intra-CSF migrated outside the CNS and reached the B-cell zone of cervical lymph nodes. However, there is yet no information on the migratory behavior of CSF-circulating DCs under neuroinflammatory conditions.
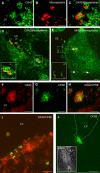
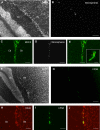
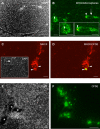
Methodology/principal findings: To address this issue, we performed in vivo transfer experiments in rats suffering from experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis. EAE or control rats were injected intra-CSF with bone marrow-derived myeloid DCs labeled with the fluorescent marker carboxyfluorescein diacetate succinimidyl ester (CFSE). In parallel experiments, fluorescent microspheres were injected intra-CSF to EAE rats in order to track endogenous antigen-presenting cells (APCs). Animals were then sacrificed on day 1 or 8 post-injection and their brain and peripheral lymph nodes were assessed for the presence of microspheres(+) APCs or CFSE(+) DCs by immunohistology and/or FACS analysis. Data showed that in EAE rats, DCs injected intra-CSF substantially infiltrated several compartments of the inflamed CNS, including the periventricular demyelinating lesions. We also found that in EAE rats, as compared to controls, a larger number of intra-CSF injected DCs reached the cervical lymph nodes. This migratory behavior was accompanied by an accentuation of EAE clinical signs and an increased systemic antibody response against myelin oligodendrocyte glycoprotein, a major immunogenic myelin antigen.
Conclusions/significance: Altogether, these results indicate that CSF-circulating DCs are able to both survey the inflamed brain and to reach the cervical lymph nodes. In EAE and maybe multiple sclerosis, CSF-circulating DCs may thus support the immune responses that develop within and outside the inflamed CNS.
Conflict of interest statement
Figures

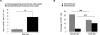




References
-
- Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. - PubMed
-
- Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, et al. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. - PubMed
-
- Pashenkov M, Teleshova N, Kouwenhoven M, Smirnova T, Jin YP, et al. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J Neuroimmunol. 2002;122:106–116. - PubMed
-
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, et al. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol. 2006;65:124–141. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

