Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation
- PMID: 18830417
- PMCID: PMC2556242
- DOI: 10.1172/JCI36245
Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation
Abstract
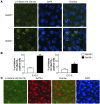
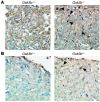
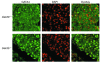
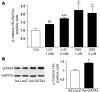
Based on extensive preclinical data, glycogen synthase kinase-3 (GSK-3) has been proposed to be a viable drug target for a wide variety of disease states, ranging from diabetes to bipolar disorder. Since these new drugs, which will be more powerful GSK-3 inhibitors than lithium, may potentially be given to women of childbearing potential, and since it has controversially been suggested that lithium therapy might be linked to congenital cardiac defects, we asked whether GSK-3 family members are required for normal heart development in mice. We report that terminal cardiomyocyte differentiation was substantially blunted in Gsk3b(-/-) embryoid bodies. While GSK-3alpha-deficient mice were born without a cardiac phenotype, no live-born Gsk3b(-/-) pups were recovered. The Gsk3b(-/-) embryos had a double outlet RV, ventricular septal defects, and hypertrophic myopathy, with near obliteration of the ventricular cavities. The hypertrophic myopathy was caused by cardiomyocyte hyperproliferation without hypertrophy and was associated with increased expression and nuclear localization of three regulators of proliferation - GATA4, cyclin D1, and c-Myc. These studies, which we believe are the first in mammals to examine the role of GSK-3alpha and GSK-3beta in the heart using loss-of-function approaches, implicate GSK-3beta as a central regulator of embryonic cardiomyocyte proliferation and differentiation, as well as of outflow tract development. Although controversy over the teratogenic effects of lithium remains, our studies suggest that caution should be exercised in the use of newer, more potent drugs targeting GSK-3 in women of childbearing age.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

