Involvement of calpain in the process of Jurkat T cell chemotaxis
- PMID: 18831007
- PMCID: PMC2678561
- DOI: 10.1002/jnr.21882
Involvement of calpain in the process of Jurkat T cell chemotaxis
Abstract
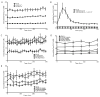
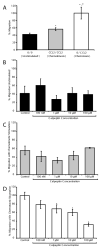
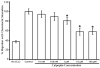
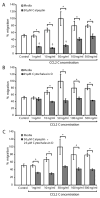
Massive T cell infiltration into the central nervous system is a hallmark of multiple sclerosis (MS) and its rodent model experimental autoimmune encephalomyelitis (EAE), resulting in the induction of many of the pathophysiological events that lead to neuroinflammation and neurodegeneration. Thus, blocking T cell migration into the central nervous system may reduce disease severity in MS and EAE. One potential target for reducing T cell migration is inhibition of the Ca(2+)-activated neutral protease calpain. Previous studies in other cell types have demonstrated that migration is reduced by incubation of cells with calpain inhibitors. Thus, we hypothesize that calpain inhibition will reduce migration of T cells in response to and toward the chemokine CCL2. To test this hypothesis, the intracellular free Ca(2+) levels in Jurkat E6-1 T cells was first measured by the fura-2 assay to assess whether the intracellular ion environment would support calpain activation. The intracellular free Ca(2+) levels were found to increase in response to CCL2. The cells were next treated with the calpain inhibitor calpeptin in a multiwelled Boyden chamber with CCL2 used as the chemoattractant. These studies demonstrate that inhibition of calpain with its inhibitor calpeptin produces a dose-dependent inhibition of chemotaxis. Calpain activity, as measured by live cell imaging, was also increased in response to CCL2, providing further evidence of its involvement in the process of chemotaxis and migration. These studies provide evidence for the involvement of calpain in the mechanisms of chemotaxis and warrants further exploration in MS patient and EAE animal samples.
(c) 2008 Wiley-Liss, Inc.
Figures

References
-
- Colvin RA, Campanella GSV, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–30227. - PubMed
-
- Cope AP, Schulze-Koops H, Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25(5 Suppl 46):S4–S11. - PubMed
-
- Das A, Sribnick EA, Wingrave JM, Del Re AM, Woodward JJ, Appel SH, Banik NL, Ray SK. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

