Role of the Zn1 and Zn2 sites in metallo-beta-lactamase L1
- PMID: 18831550
- PMCID: PMC2678235
- DOI: 10.1021/ja8035916
Role of the Zn1 and Zn2 sites in metallo-beta-lactamase L1
Abstract
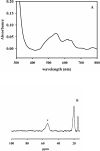
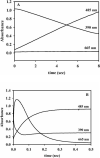
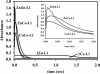
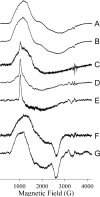
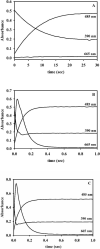
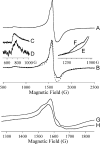
In an effort to probe the role of the Zn(II) sites in metallo-beta-lactamase L1, mononuclear metal ion containing and heterobimetallic analogues of the enzyme were generated and characterized using kinetic and spectroscopic studies. Mononuclear Zn(II)-containing L1, which binds Zn(II) in the consensus Zn1 site, was shown to be slightly active; however, this enzyme did not stabilize a nitrocefin-derived reaction intermediate that had been previously detected. Mononuclear Co(II)- and Fe(III)-containing L1 were essentially inactive, and NMR and EPR studies suggest that these metal ions bind to the consensus Zn2 site in L1. Heterobimetallic analogues (ZnCo and ZnFe) analogues of L1 were generated, and stopped-flow kinetic studies revealed that these enzymes rapidly hydrolyze nitrocefin and that there are large amounts of the reaction intermediate formed during the reaction. The heterobimetallic analogues were reacted with nitrocefin, and the reactions were rapidly freeze quenched. EPR studies on these samples demonstrate that Co(II) is 5-coordinate in the resting state, proceeds through a 4-coordinate species during the reaction, and is 5-coordinate in the enzyme-product complex. These studies demonstrate that the metal ion in the Zn1 site is essential for catalysis in L1 and that the metal ion in the Zn2 site is crucial for stabilization of the nitrocefin-derived reaction intermediate.
Figures



References
-
- Fisher JF, Meroueh SO, Mobashery S. Chem. Rev. 2005;105:395–424. - PubMed
-
- Crowder MW, Spencer J, Vila AJ. Acc. Chem. Res. 2006;39(10):721–728. - PubMed
-
- Moran-Barrio J, Gonzalez JM, Lisa MN, Costello AL, Dal Peraro M, Carloni P, Bennett B, Tierney DL, Limansky AS, Viale AM, Vila AJ. J. Biol. Chem. 2007;282:18286–18293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

