Adalimumab improves health-related quality of life in patients with moderate to severe plaque psoriasis compared with the United States general population norms: results from a randomized, controlled Phase III study
- PMID: 18831744
- PMCID: PMC2579425
- DOI: 10.1186/1477-7525-6-75
Adalimumab improves health-related quality of life in patients with moderate to severe plaque psoriasis compared with the United States general population norms: results from a randomized, controlled Phase III study
Abstract
Objective: To evaluate the impact of adalimumab on health-related quality of life (HRQOL) for patients with moderate to severe plaque psoriasis.
Background: Psoriasis is a chronic, inflammatory, immune-mediated disease that has a significant impact on patients' HRQOL. Adalimumab is a fully human monoclonal antibody that blocks tumor necrosis factor, a pro-inflammatory cytokine, and is effective and well-tolerated for patients with moderate to severe psoriasis.
Methods: Data were obtained for a secondary analysis of patients in a randomized, controlled Phase III trial evaluating the effect of adalimumab in patients with psoriasis (N = 1,205). Patients with moderate to severe psoriasis were randomized in a 2:1 ratio to adalimumab 80 mg (two 40 mg injections administered subcutaneously at baseline followed by one 40 mg injection every other week from Week 1 to Week 15) or placebo. Short Form-36 (SF-36) Health Survey scores of psoriasis patients were used to assess HRQOL and were compared with United States (US) population norms at baseline and Week 16.
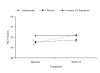
Results: Baseline Physical Component Summary (PCS) scores for the placebo and adalimumab groups were similar to the general US population. Baseline mean Mental Component Summary (MCS) scores were significantly lower for the adalimumab and placebo groups compared with the general population (47.4, 47.7, and 50.8 points, respectively; p < 0.0001). PCS scores at Week 16 for patients receiving adalimumab had improved and were significantly greater than scores for the general US population (52.7 vs 48.9; p < 0.001). Compared with the general US population, MCS scores at Week 16 were similar for patients receiving adalimumab (51.2 vs 50.8; p = 1.000) and lower for patients receiving placebo (50.8 vs 48.7; p < 0.0001).
Conclusion: Psoriasis has a broad impact on patient functioning and well-being. Improvement in skin lesions and joint symptoms associated with adalimumab treatment was accompanied by improvements in HRQOL to levels that were similar to or greater than those of the general US population.
Trial registration: Clinicaltrials.gov NCT00237887.
Figures

References
-
- Psoriasis: More Than Cosmetic http://www.fda.gov/fdac/features/2004/504_psoriasis.html
-
- de Arruda LH, De Moraes AP. The impact of psoriasis on quality of life. Br J Dermatol. 2001;144:33–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

